Abstract
65-year-old woman was admitted to our hospital with acute decompensated heart failure with reduced left ventricular ejection fraction and severe mitral regurgitation. Electrocardiography revealed a typical left bundle branch block and atrial fibrillation. Her condition deteriorated despite administering high-doses of inotropes and vasopressors. Pending a decision to therapy, venoarterial extracorporeal membrane oxygenation (ECMO) was performed when the patient underwent a cardiogenic shock. Although the hemodynamic status stabilized with ECMO support, weaning the patient from ECMO was not possible. Thus, we decided to perform cardiac resynchronization with defibrillator implantation as a “rescue” therapy. Five days post-implantation, the patient was successfully weaned from ECMO.
Cardiac resynchronization therapy (CRT) is recommended for patients on optimal medical treatment, suffering from symptomatic chronic heart failure (HF) with severely depressed left ventricular (LV) ejection fraction (EF) (≤35%) and QRS duration >120 msec.1)2) However, uncertainty remains whether it is beneficial to implant CRT devices in patients with severe HF, especially those in cardiogenic shock or end-stage HF, since such patients have been excluded from most CRT trials. Few cases have been reported about CRT implantation in patients with acute decompensated HF being treated with vasopressors/inotropes,3)4)5)6) but patients in the intensive care unit requiring mechanical circulatory support were usually not considered as eligible for CRT or CRT-implantable cardioverter defibrillator (CRT-D) “salvage” therapy. In this report, we present a case of successful extracorporeal membrane oxygenation (ECMO) weaning in a cardiogenic shock patient after CRT-D implantation.
A 65-year-old woman with dyspnea was admitted to our hospital. Two years prior, she was diagnosed with a rheumatic valvular heart disease with moderate mitral stenosis and mild mitral regurgitation (MR). Over the years, her HF symptoms progressed and left ventricular ejection fraction (LVEF) worsened, despite guideline-directed optimal medical treatment, including angiotensin-converting enzyme inhibitors, diuretics and beta blockers. One year prior to admission, coronary computed tomography angiography was performed to rule out myocardial ischemia; significant stenosis was not noted. Echocardiography showed severe LV dysfunction with an LVEF of 25%, and her LV end-diastolic diameter was 72 mm (Fig. 1A and Supplementary Video 1 in the online-only Data Supplement). Severe MR was noted, which was mainly due to dilation of the LV. The effective regurgitant orifice of the mitral valve was 45 mm2, and the regurgitation volume was 43.6 mL. Dyssynchronous cardiac motion was also observed on echocardiography. Twelve-lead electrocardiogram (ECG) revealed atrial fibrillation, left bundle branch block, and prolonged QRS duration of 141 ms (Fig. 2A). After admission, HF was aggravated and pulmonary edema (Fig. 3A) developed, along with acute kidney injury. Although high doses of dopamin, and norepinephrine were continuously infused, the blood pressure plummeted to 63/41 mmHg and heart rate (HR) was 105 bpm. Ischemic colitis developed with prolonged low cardiac output status. Mechanical circulatory support was started using venoarterial (VA) ECMO via cannulation of the femoral artery and vein. The patient's hemodynamic status stabilized with ECMO support having a flow rate of 2.5-3.0 L/min.
Volume overload, dyssynchrony, and increased MR are known to aggravate HF; ultrafiltration and continuous renal replacement therapy were therefore performed. However, there was no improvement in hemodynamic status even after 8 L of body fluid was removed. Echocardiography revealed persistent severe MR and a dilated inferior vena cava with low collapsibility, which are consistent with increased central venous pressure. Despite mechanical circulatory support with ECMO for 6 days, tachycardia (maximum HR rate 150 bpm) persisted, and ECMO flow was increased to 3.3 L/min. Amiodarone was started to control rapid ventricular response. Although the ventricular rate reduced to 70-80 bpm after amiodarone infusion, the ECMO flow could not be reduced below 2.9 L/min.
Biventricular pacing with atrioventricular (AV) node ablation was simultaneously performed while the patient was still under ECMO support, using a Quadra Assura™ CRT-D device (St. Jude Medical, St. Paul, MN, USA). Using a 0.14 inch guide wire, a quadripolar LV lead (Quartet™ lead; St. Jude Medical, St. Paul, MN, USA) was placed in the lateral branch of the coronary sinus after superselecting the cardiac vein. The selection of coronary vein was performed to enable the longest electrical delay. Under fluoroscopic guidance, a single-coil implantable cardioverter defibrillator lead was positioned in the apical septum. A right atrial lead was not implanted due to atrial fibrillation. We ablated the bundle at the His region, using a 3.5 mm tip deflectable ablation catheter. Radiofrequency energy at 25-30 watts was applied to the bundle. AV block was noted after AV node ablation, and complete biventricular capture was achieved. The first LV lead threshold was 2.25 V/0.5 ms and the impedance was 290 ohm; the right ventricular lead threshold was 0.75 V/0.5 ms and the impedance was 380 ohm. Each pacing output was 3.0 V, 2.5 V. Biventricular pacing was done over 80 bpm. The lower boundary ventricular tachycardia zone was programmed to 330 ms (181 bpm), while the ventricular fibrillation zone was set to 270 ms (222 bpm).
LV systolic function recovered, and chamber size decreased, immediately after implantation of the CRT device. The inferior vena cava decreased in size with high collapsibility; however, the amount of MR did not change compared to the previous examination. After five days, pulmonary edema and cardiomegaly improved (Fig. 3B). ECMO weaning was successful, and LV function and chamber size were further improved on predischarge evaluation: QRS duration was 132 msec (Fig. 2B), LVEF 42%, and LV end-systolic dimension 60 mm (Fig. 1B and Supplementary Video 2 in the online-only Data Supplement). The patient was discharged under treatment, after prescribing angiotensin II receptor blocker, diuretics, and beta blockers; there were no further recurrences of cardiogenic shock or symptoms of HF (Supplementary Fig. 1 in the online-only Data Supplement).
Multiple randomized clinical trials (RCTs) have shown CRT to be an effective treatment for HF patients with reduced EF and increased QRS duration. However, patients with advanced HF being managed with inotropic or percutaneous mechanical circulatory support, and patients with atrial fibrillation, were rarely included in these RCTs.1) The reasons for exclusion being a high risk of complications and short life expectancy. Therefore, the clinical usefulness of CRT in these patients remains unclear. There have been few selective studies and case reports investigating the usefulness of “rescue” CRT in patients with non-ambulatory New York Heart Association (NYHA) functional class IV HF or cardiogenic shock.3)4)5)6)7) Most attempts, however, have been unsuccessful. For example, in a retrospective study by Mantziari et al3) 24 patients underwent CRT implantation during hospitalization in the cardiac critical care unit. Six of these patients supported by an intra-aortic balloon pump were able to be weaned from mechanical circulatory support after CRT implantation. Two patients did not survive weaning from ECMO after CRT implantation.3) One pediatric dilated cardiomyopathy case reported weaning from ECMO after CRT implantation; however, intensive care unit treatment was continued due to multiple organ dysfuction.8)
Since our case was categorized as a non-ambulatory NYHA functional class IV, it was unclear whether CRT would be helpful. Heart transplantation was also considered, but was not readily available. Broad QRS duration, left bundle branch block,9) a nonischemic cause of HF, and significant dyssynchrony confirmed by echocardiography10) were favorable factors in this case. Thus, we implanted a CRT-D. Because biventricular pacing is ineffective in patients with atrial fibrillation and intact AV conduction due to fast and irregular ventricular response, AV node ablation was also performed during CRT-D implantation.
In general, CRT is useful for restoring cardiac synchrony and LV function, and for inducing LV reverse remodeling. The device also helps to reduce LV end-diastolic and end-systolic volume and MR.11) The effects of CRT gradually appear over several months in most cases. Hence, we were unsure whether ECMO could stably support the patient until she recovered. A desirable, early hemodynamic effect of CRT immediately after the procedure12) can facilitate weaning from pharmacological and mechanical support in end-stage HF.5) As expected, echocardiography revealed rapidly improving LV synchrony and contractility after implantation of CRT, with stabilization of the blood pressure and HR. The patient was successfully weaned from ECMO. Moreover, MR improved gradually to ‘moderate’ over 3 months, without resorting to cardiac surgery. This current case might therefore represent the first successful “rescue” CRT with AV node ablation in an adult patient supported by ECMO due to cardiogenic shock, who was potentially eligible for heart transplantation.
Controversy remains regarding which patients are the best candidates for ‘rescue CRT’. The response of patients undergoing biventricular pacing depends on several factors, including age, sex, underlying heart disease (cause and severity), comorbid illness, type of branch block, QRS duration, degree of dyssynchrony and the presence or absence of atrial fibrillation. As already stated, our patient had favorable characteristics, including broad QRS duration, left bundle branch block, nonischemic cause of HF, and significant dyssynchrony.9)10) Unfavorable characteristics included atrial fibrillation,13) valvular heart disease and ECMO support. CRT implantation was performed with the hope that functional MR would improve after resynchronization therapy. Consequently, careful decisions regarding whether CRT would be beneficial in all critically ill patients, are still at the discretion of the clinician. Further studies will be needed to establish better clinical decisions in such cases.
It is known that HR reductions caused by catheter ablation might improve prognosis. There have been many reports suggesting that increased HR in patients with HF is associated with an increased risk of mortality and morbidity.14) The Systolic Heart Failure treatment with the Ivabradine Trial (SHIFT) was a randomized trial of ivabradine versus placebo, in patients with symptomatic HF associated with reduced EF, sinus rhythm, and a resting HR of 70 bpm or higher.15) Patients with HR higher than 70 are at increased risk for cardiovascular death and hospital admission for worsening HF.15) In our case, the resting HR was maintained at 70 to 90 bpm. However, atrial fibrillation developed rapidly and intermittently, despite optimal medication. HR was maintained at 80 bpm after AV node ablation. Reductions in HR might increase hemodynamic stability and induce weaning from mechanical support.
In conclusion, our case study suggests that CRT with AV node ablation could be useful in selecting non-ambulatory NYHA functional class IV HF patients supported by ECMO, and the effects of the procedure could facilitate weaning from mechanical support.
Figures and Tables
Fig. 1
Comparison of serial echocardiography images in end systolic phase. (A) Apical four-chamber view and parasternal long axis view of transthoracic echocardiography, showing severe left ventricular systolic dysfunction, all-chamber dilatation, and dyssynchronous cardiac motion. (B) Apical four-chamber view and parasternal long axis view of transthoracic echocardiography showing improved LV systolic function and LV dilatation dramatically with cardiac resynchronization therapy with defibrillator. LV: left ventricular.
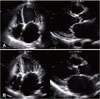
Fig. 2
Two 12-lead electrocardiogram ECG taken before and after procedure. (A) ECG taken for the first time. It revealed atrial fibrillation, complete left-bundle branch block, and QRS duration of 141 msec. (B) 12-lead electrocardiogram taken after the procedure. The ECG shows every QRS complex is paced by the device. The QRS duration decreased to 132 msec. ECG: electrocardiogram.
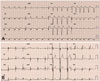
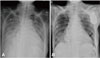
Fig. 3
Chest radiography images performed before and after CRT-D implantation. (A) Before CRT-D implantation: cardiomegaly and pulmonary edema are seen even during extracorporeal membrane oxygenation support. (B) Postoperative chest radiography showing the CRT-D device: left ventricular endocardial pacing leads are inserted (black arrow). CRT-D: cardiac resynchronization therapy with defibrillator.
References
1. European Society of Cardiology (ESC). European Heart Rhythm Association (EHRA). Brignole M, et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Europace. 2013; 15:1070–1118.
2. National Institute for Health and Care Excellence. Implantable cardioverter defibrillators and cardiac resynchronisation therapy for arrhythmias and heart failure [Internet]. London (UK): 2014. cited 2014 June 24. Available from: https://www.nice.org.uk/guidance/ta314.
3. Mantziari L, Guha K, Senguttuvan NB, Sharma R. Cardiac resynchronization therapy for critically ill patients with left ventricular systolic dysfunction. Int J Cardiol. 2013; 163:141–145.
4. Sokal A, Jedrzejczyk E, Lenarczyk R, et al. Efficacy of cardiac resynchronisation therapy in the treatment of end-stage inotrope-dependent heart failure patients. Kardiol Pol. 2014; 72:777–782.
5. Zaeem F, Giedriemiene D, Coleman C, et al. CRT-D Therapy in Patients with Decompensated NYHA Class-Four CHF. Cardiol Res Pract. 2012; 2012:319205.
6. Bhattacharya S, Abebe K, Simon M, Saba S, Adelstein E. Role of cardiac resynchronization in end-stage heart failure patients requiring inotrope therapy. J Card Fail. 2010; 16:931–937.
7. Pecha S, Yildirim Y, Reichenspurner H, Deuse T. Successful extracorporeal membrane oxygenation weaning after cardiac resynchronization therapy device implantation in a patient with end-stage heart failure. Interact Cardiovasc Thorac Surg. 2012; 15:922–923.
8. Bang JH, Oh YN, Ko JK, Kang SY, Baek JS, Park CS. Cardiac Resynchronization Therapy in Infant with Dilated Cardiomyopathy during Extracorporeal Membrane Oxygenator. Korean J Thorac Cardiovasc Surg. 2015; 48:55–58.
9. Cowburn PJ, Patel H, Jolliffe RE, Wald RW, Parker JD. Cardiac resynchronization therapy: an option for inotrope-supported patients with end-stage heart failure? Eur J Heart Fail. 2005; 7:215–217.
10. Serri K, Lafitte S, Amyot R, Sauve C, Roudaut R. Echocardiographic evaluation of cardiac dyssynchrony. Can J Cardiol. 2007; 23:303–310.
11. Moss AJ, Hall WJ, Cannom DS, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009; 361:1329–1338.
12. Leclercq C, Cazeau S, Le Breton H, et al. Acute hemodynamic effects of biventricular DDD pacing in patients with end-stage heart failure. J Am Coll Cardiol. 1998; 32:1825–1831.
13. Upadhyay GA, Choudhry NK, Auricchio A, Ruskin J, Singh JP. Cardiac resynchronization in patients with atrial fibrillation: a meta-analysis of prospective cohort studies. J Am Coll Cardiol. 2008; 52:1239–1246.
14. Pocock SJ, Wang D, Pfeffer MA, et al. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J. 2006; 27:65–75.
15. Swedberg K, Komajda M, Böhm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010; 376:875–885.
Supplementary Materials
The online-only Data Supplements are available with article at https://doi.org/10.4070/kcj.2016.0176.
Supplementary Fig. 1
The first days of starting mechanical support regarded 1 day. ECMO flow began to decelerate gradually, right after insertion. However the flow has risen on the seventh day after ECMO insertion. CRT-D was implanted on day 8 after ECMO insertion. After five days of CRT-D implantation, ECMO was weaned successfully. SBP: systolic blood pressure, DBP: diastolic blood pressure, HR: heart rate, ECMO: extracorporeal membrane oxygenation, CRRT: continuous renal replacement therapy, CRT-D: cardiac resynchronization therapyimplantable cardioverter defibrillator.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download