Abstract
Carotid ultrasound is an imaging modality that allows non-invasive assessment of vascular anatomy and function. Carotid intima-media thickness (IMT) has been shown to predict cardiovascular (CV) risk in multiple large studies. However, in 2013, American College of Cardiology/American Heart Association guidelines designated that the carotid IMT as class III evidence level was not recommended for use in clinical practice as a routine measurement of risk assessment for a first atherosclerotic CV event. Following the announcement of this guideline, combined common carotid IMT and plaque, including plaque tissue characterization and plaque burden, using 3D ultrasound was reported to be better than either measurement alone in a variety of studies. Moreover, changes in the intima thickness were related to aging and early atherosclerosis, and remodeling of the media thickness was associated with hypertension. Separate measurement is useful for evaluating the effects of different atherosclerotic risk factors on the arterial wall; however, a more detailed and elaborate technique needs to be developed. If so, separate measurement will play an important role in the assessment of atherosclerosis and arterial wall change according to a variety of risk factors, such as metabolic syndrome. In addition, although carotid blood flow velocity is a useful tool for risk classification and prediction in clinical practice, further clinical research is needed. The value of carotid IMT by ultrasound examination for risk stratification remains controversial, and groups developing future guidelines should consider the roles of plaque presence and burden and hemodynamic parameters in additional risk stratification beyond carotid IMT in clinical practice.
Carotid ultrasound is one of the several imaging modalities that allow non-invasive assessment of vascular anatomy and function.1)2) Use of this technique allows measurement of a variety of parameters including intima-media thickness (IMT), arterial diameter, the presence of plaque, blood flow and velocity measurements. The typical B-mode image of the arterial wall is defined as the double line pattern, in which the inner line is generated by the intima surface, and this imaging approach is a useful and safe tool for the measurement of IMT.3) In addition, the consensus statement from the American Society of Echocardiography has simplified carotid IMT and plaque assessment methodology.4)
Carotid IMT and presence of plaque have been shown to predict cardiovascular events in multiple large studies.5)6)7)8) Also, in low-risk subjects, initial screening by IMT and plaque assessment is likely to provide useful information for the detection of subclinical atherosclerosis.9) Furthermore, common carotid blood flow (CBF) velocity was independently associated with future cardiovascular disease (CVD) using color duplex ultrasound and Doppler spectral analysis.10) In clinical practice, evaluation of the carotid artery by ultrasonography is a very useful, simple, and safe method to indirectly detect and prevent CVD.
However, in 2013, American College of Cardiology/American Heart Association (ACC/AHA) guidelines for cardiovascular risk assessment designated that the carotid IMT as class III evidence level was not recommended for use in clinical practice as a routine measurement of risk assessment for a first atherosclerotic cardiovascular disease (ASCVD) event.11) After the announcement of this guideline, Naqvi et al.12) reported that the controversy surrounding the usefulness of carotid IMT measurement in risk stratification appears to result from the lack of a uniform methodology in carotid IMT studies.
This article reviews the studies performed after the announcement of this guideline and which evaluated carotid IMT for ASCVD risk prediction and risk estimation in a specific condition. In addition, this article discusses the value of common carotid IMT (CCA-IMT) and plaque parameters in CVD risk assessment and prediction. The article summarizes the studies using hemodynamic parameters of the carotid artery in CVD risk association or prediction and also discusses the separate measurement of carotid IMT and its usefulness and limitations in clinical practice.
Carotid IMT is used to measure the distance between the luminal border of the intima and the outer border of the media of the carotid artery far wall, which is represented as a double-line pattern on a B-mode ultrasound image (Fig. 1).3)4) The carotid artery includes four segments: common carotid artery (CCA), bifurcation (bulb), external carotid artery and internal carotid artery (ICA). The IMT can be measured in the CCA, bulb, and ICA.13) Among the limitations frequently cited in measurement of carotid IMT is the difficulty in appropriately imaging all carotid artery segments, especially the ICA-IMT.
In the Atherosclerosis Risk in Communities (ARIC) study, 91.4% of the CCA-IMT segments could be adequately imaged compared with 77.3% of bulb IMT segments and 48.6% of ICA-IMT segments.5) Similarly, in the Rotterdam study, carotid IMT measurements were possible in 96% of the CCA-IMT segments compared with 64% of bulb-IMT segments and 31% of ICA-IMT segments.14)
Considering accuracy and best reproducibility, CCA far wall IMT measurement was validated as representing the true thickness of the vessel wall.13) The development of automated edge detection programs for use instead of manual measurements has increased the speed and reduced the variability of measurements.15)
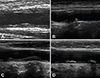
Carotid IMT is the sum of the intima thickness (IT) and the media thickness (MT) (Fig. 1). In an animal study, the carotid high-echogenic intimal thickening (HEIT) examined using a 40 MHz correlates closely with the histological IT (Fig. 2).16) Separate measurements of the individual intima and media layers in carotid arteries can be examined using high-resolution B-mode ultrasound.17) Remodeling of the thickness of the intima and media layers is a distinct process that exhibits independent changes in different types of arteries with advancing age and throughout the development of atherosclerosis.18) Early vascular changes caused by atherosclerosis occur primarily in the IT.17) Thus, the IT can be a useful parameter for early detection, prediction of progression and evaluation of atherosclerosis.19) Increased IMT in hypertensive patients is caused by increased MT and is compatible with medial hypertrophy seen in hypertension.20) Therefore, separate measurements are useful for assessing the effects of different atherosclerotic risk factors on the arterial wall.21)
However, the theoretical axial resolution of a 7 MHz transducer is approximately 0.3 mm, and the high-frequency (about 11-15 MHz transducer) ultrasound pixel resolution is approximately 0.1 to 0.2 mm.22) Therefore, if the IMT complex is thinner than 0.3 mm, the leading edges of the two echo interfaces from far wall intima and adventitia respectively cannot be separated, and measurement of the intima–media complex is not possible using a 7 MHz transducer.22) Furthermore, the mean IT is approximately 0.2 mm and it can be intermittently or inadequately measured even by using a high-frequency transducer. This limitation should be the focus of attention in future studies. In addition, the annual change of carotid IMT value is approximately 0.01 to 0.04 mm per year in the general population23) and patients with disease;24) this is lower than the current resolution of ultrasound. Therefore, it is impossible to analyze carotid IMT change over a short period.
A meta-analysis of 14 population-based studies that evaluated CCA-IMT alone and excluded CCA, bulb IMT and plaque showed that the addition of CCA-IMT measurement to the Framingham Risk Score was associated with small improvement in 10-year risk prediction of first-time myocardial infarction or stroke, but this improvement is unlikely to be of clinical importance.6)25) However, CCA-IMT including the carotid bulb and ICA-IMT were found to be better predictors of both cardiac risk and stroke risk.26) Carotid plaque appears to be a more powerful predictor of CV risk than carotid IMT alone.27) In summary, many previous studies have revealed discrepancies in the comprehensiveness with which carotid IMT was assessed with regard to the number of carotid segments evaluated (CCA, ICA, or the carotid bulb), the type of measurements (mean or maximum of single measurements, mean of the mean, or mean of the maximum for multiple measurements), whether plaques were included in the IMT measurement, use of adjusted or unadjusted models, risk association versus risk prediction, and arbitrary cutoff points for CIMT and plaque to predict risk.12)
After the announcement of the 2013 ACC/AHA guidelines for CV risk assessment,11) the ARIC study reported that coronary heart disease (CHD) risk prediction can be improved by adding all carotid artery segments (A-CIMT) including the presence of plaque or CCA-IMT and plaque information to traditional risk factors (TRF) compared with CCA-IMT alone. Furthermore, because measurement of CCA-IMT is easier and more reliable than A-CIMT, evaluating the carotid artery for plaque presence and measuring CCA-IMT provide a good parameter for CHD risk prediction.28)
In a recent guideline, the carotid IMT was not recommended for use in clinical practice as a routine measurement of risk assessment for a first ASCVD event. Thus, it is unclear when carotid IMT should be measured in clinical practice. In a recent study, carotid plaque was found to be more useful as an additive predictive factor for primary prevention of ASCVD than CCA-IMT alone in asymptomatic high risk patients.29) CCA-IMT and carotid plaque were useful prognostic parameters to predict long-term future CV events in patients with well-treated ST elevation myocardial infarction (MI). The value of CCA-IMT measurement in predicting CV events seems to be of clinical importance beyond the TRF in this relatively low-risk population with acute MI.30) In patients with one or more TRF, carotid plaque is more useful as an additive predictive factor for primary and secondary prevention of ASCVD than is CCA-IMT alone.31)
In a recent study with a small sample size, no significant differences in clinical outcomes from CV events, including death, MI, and stroke were observed between the highest and lowest CCA-IMT values based on the inter-quartile range in the younger subjects (males <45 years and female <55 years) with hypertension.32) Another study reported similar results, showing that, within a relatively young Iranian population of individuals without a history of CV event, thicker carotid IMT did not associate with several modifiable CV risk factors.33)
In the elderly hypertensive population aged at least 60 years, only calcified carotid plaques (except for mean A-CIMT) predicted mortality and cardiovascular outcomes above other traditional CVD risk factors such as age, sex, and hypertensive status.34)
Carotid plaque is identified as an echoic focal projection, or as the presence of focal wall thickening that is at least 50% greater than that of the surrounding vessel wall or as a focal region with carotid IMT greater than 1.5 mm that protrudes into the lumen and is distinct from the adjacent boundary.4) Other definitions for plaque identification include shadowing in wall texture, roughness, and inconsistency in the visualization of structural boundaries together with bright echogenicity.35) Many previous studies have reported different approaches to the analysis and assessment of plaques, such as recording the absence or presence of plaque,36) the size or burden of plaque (mild, moderate, or severe),37) number of visible plaques (no, single, or multiple)38) and composition or tissue characterization (echolucent, or calcified) (Fig. 3).35)39) However, reliable characterization of plaque tissue content and features suggestive of plaque instability (ulceration, thin fibrous cap) using standard carotid ultrasound techniques is not yet possible. In addition, there is a need for a developing a molecular imaging method that is capable of identifying the echolucent or calcified plaque on carotid ultrasound.
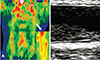
Some studies have attempted to analyze the tissue properties of plaque with ultrasound techniques using different imaging modalities. Using positron emission tomography-computerized tomography, an echolucent plaque has greater F-18 FDG uptake than calcified plaque or no plaque, which seems to imply a high inflammatory state (Fig. 4).40) Calcified regions of the carotid plaque were characterized as white radiodense areas by spiral CT imaging, in contrast to the remaining non-calcified regions of the plaque and lumen.41) Using high-resolution MRI, 71% of the carotid plaques contain a lipid core, which might provide evidence supporting more aggressive cholesterol-lowering therapy.42)
With recent developments in technology, it has become possible to analyze the ultrasound characteristics of complex plaques. Computer-assisted pixel distribution analysis of duplex ultrasound scan images accurately quantified intraplaque hemorrhage, fibromuscular tissue, calcium, and lipid.43) In addition, the emergence of 3-dimensional (3D) ultrasound allows more accurate quantification of plaque volume or area. Plaque areas from all cross-sectional images in the entire image sequence were summed as the plaque burden.44) Therefore, not only the presence or absence of plaques, but also the characteristics of plaques or plaque burden can be evaluated with regard to prognosis of CV events31) and its implications for CV risk.45)
Biomarkers are a convenient method to detect high-risk individuals, to diagnose disease conditions promptly and accurately, and to effectively evaluate the prognosis and treatment outcomes of patients with disease. The role of carotid IMT as a structural marker of the artery and as a biomarker for identifying high-risk patients has been investigated.46) According to the 2013 ACC/AHA guideline, if a risk-based treatment decision is vague after quantitative TRF assessment, evaluation of one or more of the following factors may be considered to make informed treatment decisions: family history, hs-CRP, coronary artery calcium score (CACS), or ankle-brachial index (ABI).11) Assessment of CACS, which is similar in form to a structural biomarker of the artery, is useful for diagnosis and as a surrogate marker of ASCVD compared with CCA-IMT or plaque.31) Carotid plaque and increased carotid IMT are associated with the presence and degree of coronary calcification and disease.47) ABI is a functional biomarker of the artery. In one retrospective study, patients with greater mean CCA-IMT (≥0.9 mm) or lower ABI (<0.9) had significantly higher complexity and presence of CAD. Also, the combination of CCA-IMT and ABI provides additive information for predicting the severity and presence of CAD.48) In another study, the hs-CRP level, which is a serological biomarker of the artery, and carotid plaque characteristics were found to correlate closely with the severity of CAD.49)
Carotid ultrasound is widely used to measure hemodynamic parameters such as peak-systolic velocity (PSV), end-diastolic velocity (EDV), and resistive index (RI), calculated as (PSV-EDV)/PSV (Fig. 5).
In a case-control study, stroke patients in an acute as well as a chronic stable phase appeared to have lower CBF velocity and volume and higher RI than non-stroke patients, independent of carotid atherosclerosis.50) In a Taiwanese population at low risk for atherosclerosis, CCA-IMT and EDV could jointly predict the risk of future ischemic stroke events, and EDV value was more strongly associated with ischemic stroke than was CCA-IMT.51) In addition, in a prospective study, carotid flow velocity (CFV) was significantly associated with the development of CVD during a median follow-up time of 12.8 years. CBF velocity, particularly EDV, also improved the risk prediction of CVD.10) In a total of 1,119 Korean patients without CHD or stroke, among the carotid Doppler indices, higher RI, and lower CCA-PSV and CCA-EDV, but not ICA Doppler indices, were related to future CV events.52) Therefore, CFV represents a subclinical atherosclerosis index and it should be included in the assessment of CVD risk.
Measurement of CCA-IMT and plaque detection using ultrasound imaging can be easily, simply, safely, and reproducibly accomplished in the outpatient setting as a noninvasive screening method for CV risk assessment. Considerable technical developments are needed to evaluate the characteristics or burden of plaque and to identify plaque reproducibility. Also, abnormal cutoff values for CCA-IMT, plaque presence, and size or volume, adjusted for age, race, and sex remain to be defined. In addition, the reproducibility, abnormal cutoff value, and the role of carotid hemodynamic parameters must be defined in large multicenter studies.
The controversial circumstances in which carotid ultrasound examination is performed raise questions about the usefulness of carotid IMT measurement in risk stratification. The usefulness of carotid IMT measurement in clinical practice remains uncertain. Measurements of carotid IMT at the carotid bulb and at the ICA are more useful than that of CCA-IMT, both for risk classification and risk prediction. However, in view of the accuracy and best reproducibility, CCA-IMT measurement was validated as best representing the thickness of the vessel wall. Future developments in ultrasound technology will focus on separate measurements of the carotid artery to evaluate the effects of different atherosclerotic risk factors on the arterial wall. We posit that separate measurement will play an important role in the evaluation of subclinical atherosclerosis and remodeling of the arterial wall according to a variety of risk factors, such as metabolic syndrome. Assessment of plaque burden or size is a better method of determining atherosclerosis and CV risk than is a simple assessment of the presence or absence of plaques. In addition, plaques that appear echolucent or soft on B-mode ultrasound are lipid rich, whereas echogenic plaques have a higher content of dense fibrous tissue and calcification. The characteristics of an echolucent or soft plaque might provide evidence for more aggressive therapy, although further study is needed to verify this. Combined CCA-IMT and plaque assessment, including plaque tissue characterization and plaque burden, using 3D ultrasound appears to be better than either measurement alone for the assessment and prediction of ASCVD risk. Also, further study is needed to determine the change or remodeling of CCA-IMT and plaque after therapy or intervention. In addition, although carotid hemodynamic parameters are useful tools for risk classification and risk prediction in clinical practice, additional clinical research is needed. Furthermore, plaque progression and regression assessed by 3D ultrasound may be a powerful method to assess the effect of therapy. Groups developing future guidelines should consider the roles of plaque presence and burden and hemodynamic parameters in additional risk stratification beyond carotid IMT.
Figures and Tables
Fig. 1
The double line pattern and separate measurement of carotid intima-media thickness. Carotid IMT is defined as the distance between the luminal border of the intima and the outer border of media of carotid artery far wall on a B-mode ultrasound image. (A) True longitudinal plane simultaneously demonstrating double lines on the near and far walls of the common carotid artery. (B) Separately IMT, MT, and IT measured on far walls of the common carotid artery. IMT: intima-media thickness, MT: media thickness, IT: intima thickness. Modified with permission from Kim et al.21)

Fig. 2
Comparison of HEIT and IT. (A-C) Normotensive rats. (D-F) Spontaneously hypertensive rats SHR. (A) Measurement of HEIT and IMT on UBM images in normotensive rats. Red arrow indicates HEIT, and blue arrow indicates IMT. (B) The thin layer of endothelial cells is shown at the intimal area of the carotid artery. (C) Example of the thin layer of intima stained with MT staining. (D) Measurement of HEIT and IMT on UBM images in SHR. (E) The thick layer of endothelial cells is shown at the intimal area of the carotid artery. (F) Example of a thickened intimal layer with endothelial cells is shown with MT staining. HEIT: high-echogenic intimal thickening, IT: intimal thickness, IMT: intima-media thickness, MT: Masson's trichrome, UBM: ultrasound biomicroscope, SHR: spontaneously hypertensive rat.

Fig. 3
Variable tissue characterization of carotid plaque. (A) Single or focal plaque on normal common carotid intima-media thickness. (B) Irregular surface plaque on carotid bulb. (C) Diffuse longitudinal and echolucent plaque on common carotid artery. (D) Calcified and heterogeneous plaque on common carotid artery.
References
1. Schmidt-Trucksass A, Grathwohl D, Schmid A, et al. Structural, functional, and hemodynamic changes of the common carotid artery with age in male subjects. Arterioscler Thromb Vasc Biol. 1999; 19:1091–1097.
2. Schoning M, Walter J, Scheel P. Estimation of cerebral blood flow through color duplex sonography of the carotid and vertebral arteries in healthy adults. Stroke. 1994; 25:17–22.
3. Pignoli P, Tremoli E, Poli A, Oreste P, Paoletti R. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation. 1986; 74:1399–1406.
4. Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008; 21:93–111. quiz 189-90.
5. Howard G, Sharrett AR, Heiss G, et al. Carotid artery intimal-medial thickness distribution in general populations as evaluated by B-mode ultrasound. ARIC Investigators. Stroke. 1993; 24:1297–1304.
6. Polak JF, Pencina MJ, Pencina KM, O'Donnell CJ, Wolf PA, D'Agostino RB Sr. Carotid-wall intima-media thickness and cardiovascular events. N Engl J Med. 2011; 365:213–221.
7. van der Meer IM, Bots ML, Hofman A, del Sol AI, van der Kuip DA, Witteman JC. Predictive value of noninvasive measures of atherosclerosis for incident myocardial infarction: the Rotterdam Study. Circulation. 2004; 109:1089–1094.
8. Salonen JT, Salonen R. Ultrasonographically assessed carotid morphology and the risk of coronary heart disease. Arterioscler Thromb. 1991; 11:1245–1249.
9. Naqvi TZ, Mendoza F, Rafii F, et al. High prevalence of ultrasound detected carotid atherosclerosis in subjects with low Framingham risk score: potential implications for screening for subclinical atherosclerosis. J Am Soc Echocardiogr. 2010; 23:809–815.
10. Chuang SY, Bai CH, Cheng HM, et al. Common carotid artery end-diastolic velocity is independently associated with future cardiovascular events. Eur J Prev Cardiol. 2016; 23:116–124.
11. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. JACC. 2014; 63:2935–2959.
12. Naqvi TZ, Lee MS. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging. 2014; 7:1025–1038.
13. Roman MJ, Naqvi TZ, Gardin JM, et al. Clinical application of noninvasive vascular ultrasound in cardiovascular risk stratification: a report from the American Society of Echocardiography and the Society of Vascular Medicine and Biology. J Am Soc Echocardiogr. 2006; 19:943–954.
14. del Sol AI, Moons KG, Hollander M, et al. Is carotid intima-media thickness useful in cardiovascular disease risk assessment? The Rotterdam Study. Stroke. 2001; 32:1532–1538.
15. Kanters SD, Algra A, van Leeuwen MS, Banga JD. Reproducibility of in vivo carotid intima-media thickness measurements: a review. Stroke. 1997; 28:665–671.
16. Choi YS, Youn HJ, Youn JS, Park CS, Oh YS, Chung WS. Measurement of the intimal thickness of the carotid artery: comparison between 40 MHz ultrasound and histology in rats. Ultrasound Med Biol. 2009; 35:962–966.
17. Bae JH, Kim WS, Rihal CS, Lerman A. Individual measurement and significance of carotid intima, media, and intima-media thickness by B-mode ultrasonographic image processing. Arterioscler Thromb Vasc Biol. 2006; 26:2380–2385.
18. Gussenhoven EJ, Frietman PA, The SH, et al. Assessment of medial thinning in atherosclerosis by intravascular ultrasound. Am J Cardiol. 1991; 68:1625–1632.
19. Nakashima Y, Chen YX, Kinukawa N, Sueishi K. Distributions of diffuse intimal thickening in human arteries: preferential expression in atherosclerosis-prone arteries from an early age. Virchows Arch. 2002; 441:279–288.
20. Won HK, Kim WS, Kim KY, Hyun DW, Kwon TG, Bae JH. Increased carotid intima-media thickness in hypertensive patients is caused by increased medial thickness. Korean J Med. 2008; 75:179–185.
21. Kim JH, Youn HJ, Kim GH, Moon KW, Yoo KD, Kim CM. The clinical significance of separate measurements of carotid arterial wall to assess the risk factor for atherosclerosis. J Cardiovasc Ultrasound. 2016; 24:48–54.
22. Wikstrand J. Methodological considerations of ultrasound measurement of carotid artery intima-media thickness and lumen diameter. Clin Physiol Funct Imaging. 2007; 27:341–345.
23. O'Leary DH, Polak JF, Kronmal RA, et al. Distribution and correlates of sonographically detected carotid artery disease in the Cardiovascular Health Study. The CHS Collaborative Research Group. Stroke. 1992; 23:1752–1760.
24. Hlatky MA, Greenland P, Arnett DK, et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009; 119:2408–2416.
25. Den Ruijter HM, Peters SA, Anderson TJ, et al. Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis. JAMA. 2012; 308:796–803.
26. Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis. Atherosclerosis. 2012; 220:128–133.
27. Peters SA, den Ruijter HM, Bots ML, Moons KG. Improvements in risk stratification for the occurrence of cardiovascular disease by imaging subclinical atherosclerosis: a systematic review. Heart. 2012; 98:177–184.
28. Nambi V, Chambless L, He M, et al. Common carotid artery intima-media thickness is as good as carotid intima-media thickness of all carotid artery segments in improving prediction of coronary heart disease risk in the Atherosclerosis Risk in Communities (ARIC) study. Eur Heart J. 2012; 33:183–190.
29. Kim G, Youn HJ, Choi YS, Jung HO, Chung WS, Kim CM. Is carotid artery evaluation necessary for primary prevention in asymptomatic high-risk patients without atherosclerotic cardiovascular disease? Clin Interv Aging. 2015; 10:1111–1119.
30. Lee S, Cho GY, Kim HS, et al. Common carotid intima-media thickness as a risk factor for outcomes in Asian patients with acute ST-elevation myocardial infarction. Can J Cardiol. 2014; 30:1620–1626.
31. Kim GH, Youn HJ, Choi YS, Jung HO, Chung WS, Kim CM. Carotid artery evaluation and coronary calcium score: which is better for the diagnosis and prevention of atherosclerotic cardiovascular disease? Int J Clin Exp Med. 2015; 8:18591–18600.
32. Yu JS, Choi YS, Kim JY, et al. Carotid intima-media thickness is not related with clinical outcomes in young hypertensives. Clin Hypertens. 2015; 21:15.
33. Azarpazhooh MR, Kazemi-Bajestani SM, Esmaeili H, et al. Cardiovascular risk factors and nutritional intake are not associated with ultrasound-defined increased carotid intima media thickness in individuals without a history of cardiovascular events. Int J Prev Med. 2014; 5:1412–1421.
34. Thompson T, Shields KJ, Barinas-Mitchell E, Newman A, Sutton-Tyrrell K. Calcified carotid artery plaques predict cardiovascular outcomes in the elderly. J Hypertens. 2015; 33:810–817. discussion 817.
35. Prabhakaran S, Singh R, Zhou X, Ramas R, Sacco RL, Rundek T. Presence of calcified carotid plaque predicts vascular events: the Northern Manhattan Study. Atherosclerosis. 2007; 195:e197–e201.
36. Nambi V, Chambless L, Folsom AR, et al. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (Atherosclerosis Risk In Communities) study. J Am Coll Cardiol. 2010; 55:1600–1607.
37. Peters SA, Dogan S, Meijer R, et al. The use of plaque score measurements to assess changes in atherosclerotic plaque burden induced by lipid-lowering therapy over time: the METEOR study. J Atheroscler Thromb. 2011; 18:784–795.
38. Plichart M, Celermajer DS, Zureik M, et al. Carotid intima-media thickness in plaque-free site, carotid plaques and coronary heart disease risk prediction in older adults. The Three-City Study. Atherosclerosis. 2011; 219:917–924.
39. Kim JH, Youn HJ, Hong EJ, et al. Clinical significance of B-mode ultrasound of common carotid artery for prediction of severity of coronary artery disease: important parameters on hand measurement. Korean Circ J. 2005; 35:467–473.
40. Choi YS, Youn HJ, Chung WB, et al. Uptake of F-18 FDG and ultrasound analysis of carotid plaque. J Nucl Cardiol. 2011; 18:267–272.
41. Wahlgren CM, Zheng W, Shaalan W, Tang J, Bassiouny HS. Human carotid plaque calcification and vulnerability. Relationship between degree of plaque calcification, fibrous cap inflammatory gene expression and symptomatology. Cerebrovasc Dis. 2009; 27:193–200.
42. Wasserman BA, Sharrett AR, Lai S, et al. Risk factor associations with the presence of a lipid core in carotid plaque of asymptomatic individuals using high-resolution MRI: the multi-ethnic study of atherosclerosis (MESA). Stroke. 2008; 39:329–335.
43. Lal BK, Hobson RW 2nd, Pappas PJ, et al. Pixel distribution analysis of B-mode ultrasound scan images predicts histologic features of atherosclerotic carotid plaques. J Vasc Surg. 2002; 35:1210–1217.
44. Sillesen H, Muntendam P, Adourian A, et al. Carotid plaque burden as a measure of subclinical atherosclerosis: comparison with other tests for subclinical arterial disease in the High Risk Plaque BioImage study. JACC Cardiovasc Imaging. 2012; 5:681–689.
45. Khalil A, Huffman MD, Prabhakaran D, et al. Predictors of carotid intima-media thickness and carotid plaque in young Indian adults: the New Delhi birth cohort. Int J Cardiol. 2013; 167:1322–1328.
46. Vasan RS. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation. 2006; 113:2335–2362.
47. Cohen GI, Aboufakher R, Bess R, et al. Relationship between carotid disease on ultrasound and coronary disease on CT angiography. JACC Cardiovasc Imaging. 2013; 6:1160–1167.
48. Ikeda N, Kogame N, Iijima R, Nakamura M, Sugi K. Impact of carotid artery ultrasound and ankle-brachial index on prediction of severity of SYNTAX score. Circ J. 2013; 77:712–716.
49. Liang Y, Hou Y, Niu H, Lu M, Xue L, Sun Q. Correlation of high-sensitivity C-reactive protein and carotid plaques with coronary artery disease in elderly patients. Exp Ther Med. 2015; 10:275–278.
50. Bai CH, Chen JR, Chiu HC, Pan WH. Lower blood flow velocity, higher resistance index, and larger diameter of extracranial carotid arteries are associated with ischemic stroke independently of carotid atherosclerosis and cardiovascular risk factors. J Clin Ultrasound. 2007; 35:322–330.
51. Chuang SY, Bai CH, Chen JR, et al. Common carotid end-diastolic velocity and intima-media thickness jointly predict ischemic stroke in Taiwan. Stroke. 2011; 42:1338–1344.
52. Chung H, Jung YH, Kim KH, et al. Carotid artery end-diastolic velocity and future cerebro-cardiovascular events in asymptomatic high risk patients. Korean Circ J. 2016; 46:72–78.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download