Abstract
We present an iatrogenic, pleuro-pericardial connection resulting from pericardiocentesis of a large, tuberculous, pericardial effusion. Recognition of this situation is paramount when one is unable to aspirate pericardial fluid after a successful, initial puncture. Such knowledge will help prevent myocardial or coronary artery injury with further attempts at aspiration.
Percutaneous, echocardiography-guided pericardiocentesis is a safe procedure, indicated for large or haemodynamically-significant, pericardial effusions.1)2) Tuberculosis is highly prevalent in Southern Africa, with incidence rates of >1000 new cases per 100000 per year.3) Tuberculous pericardial disease can present with pericarditis, a pericardial effusion or pericardial constriction.4) The creation of an iatrogenic, pleuro-pericardial connection is an unusual complication of the procedure mentioned above – having only been reported five times in the context of larger series, and once as a case.5)6)7)8)9)
A 16 year-old female patient presented with progressive dyspnea over three weeks, a productive cough, night sweats, and lower-limb swelling. On examination, she was hypotensive (blood pressure of 96/48 mmHg), tachycardic (heart rate of 116/minute), mildly tachypneic (respiratory rate of 20/minute), and apyrexial (temperature of 36.9 degrees Celsius). Physical examination revealed generalized lymphadenopathy, an elevated jugular venous pressure and soft heart sounds. Pulsus paradoxus was absent, and the 12-lead electrocardiogram showed a sinus tachycardia with small complexes, but no electrical alternans. Chest radiography revealed an increased cardiothoracic ratio (Fig. 1), whilst a large, circumferential, pericardial effusion (measuring 35.7 mm at end-diastole over the left ventricular free wall) was identified on transthoracic echocardiography. Echocardiographic features of tamponade were present: transmitral flow velocity variation of 34%, end-diastolic, right atrial wall collapse and a distended, non-collapsing inferior vena cava.
Percutaneous, echocardiography-guided pericardiocentesis was performed via an apical approach. A 7-F puncture kit was employed, and the needle was exchanged over a wire for a dilator, and subsequently a sheath, through which a multihole-pigtail catheter was introduced into the pericardial space. Four millilitres of serous fluid was freely aspirated with the initial needle insertion (confirming its intrapericardial position), but subsequent to placement of the sheath, no further fluid could be aspirated. Immediate echocardiography and same-day chest radiography, demonstrated the formation of a left sided pleural effusion, with complete resolution of the pericardial effusion (Fig. 2, 3). Echocardiography 4 days post-pericardiocentesis demonstrated normal systolic and diastolic function (E'lateral)=18.6 cm/s, E/A=1.62), with no signs of constrictive physiology.
Analysis of the pericardial fluid showed acid-fast bacilli, as well as an adenosine deaminase level of 68 U/l, confirming the diagnosis of tuberculous pericarditis.10) Standard, antituberculous therapy was initiated, and the patient had an uncomplicated course of hospitalization. Follow-up echocardiography revealed no reaccumulation of the pericardial effusion and normal diastolic function parameters.
The immediate disappearance of the pericardial effusion, together with the occurrence of a new, pleural effusion, in the context of percutaneous pericardiocentesis, suggests that a pleuro-pericardial connection was created iatrogenically. The large pericardial effusion would have been able to drain into the pleural space, subsequent to a pressure gradient between the pericardial space (high pressure) and the pleural space (subatmospheric pressure).9)
Since the creation of a pleuro-pericardial connection, by means of percutaneous balloon, pericardiotomy prevents reaccumulation of a pericardial effusion (at least in the short term), the risk of reaccumulation of pericardial fluid was considered negligible, and prolonged indwelling catheter drainage (with the attendant discomfort and risk of infection) was not performed.11)12) We propose the following mechanism: because the patient had pendulous mammary tissue, her left breast was elevated manually prior to needle and guidewire placement, which resulted in the cephalic displacement of the subcutaneous tissue relative to the pericardial surface. However, the breast (and subcutaneous tissue) was allowed to assume a more caudal position prior to the placement of the dilator, which meant that the entry point of the guidewire into the skin and subcutaneous tissue was more inferior than the site of pericardial puncture. Upon insertion of the rigid dilator, the wire was possibly pulled vertically through the pericardial sac in an area of pleuro-pericardial overlap.
We emphasize the importance of recognizing this clinical situation in order to avoid repeated attempts at aspiration of an empty pericardial space, after the pericardial fluid had drained into the pleural space which could potentially lead to myocardial or coronary artery injury.9) It is therefore imperative to consider this complication – albeit rare – and perform echocardiography when fluid cannot be aspirated after the pericardial puncture. This complication can possibly be prevented by careful fluoroscopic and echocardiographic guidance of needle insertion.
An iatrogenic, pleuro-pericardial connection is a very rare complication of percutaneous pericardiocentesis, and since only a few cases have been documented, it is unknown whether it is more commonly encountered in certain etiologies. This is the first reported case in the context of a tuberculous, pericardial effusion.
In conclusion, iatrogenic, pleuro-pericardial connection is a rare complication of percutaneous pericardiocentesis. It should be considered when unsuccessful aspiration is encountered after successful pericardial puncture, and investigated with echocardiography to prevent myocardial or coronary artery trauma with further attempts at aspiration.
Figures and Tables
Fig. 1
Chest radiograph, pre-pericardiocentesis, demonstrating the increased cardiothoracic ratio, suggesting the presence of a large pericardial effusion.
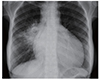
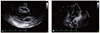
Fig. 2
Post-pericardiocentesis echocardiography. (A) Two-dimensional echocardiogram (parasternal, long-axis view), performed immediately post-pericardiocentesis, showing complete drainage of the pericardial effusion. (B) Two-dimensional echocardiogram (apical, 4-chamber view), performed immediately post-pericardiocentesis, showing a significant pleural effusion. A sliver of pericardial fluid can still be appreciated.
References
1. Maisch B, Seferovic PM, Ristic AD, et al. Guidelines on the diagnosis and management of pericardial diseases executive summary; the task force on the diagnosis and management of pericardial diseases of the European society of cardiology. Eur Heart J. 2004; 25:587–610.
2. Tsang TS, Enriquez-Sarano M, Freeman WK, et al. Consecutive 1127 therapeutic echocardiographically guided pericardiocenteses: clinical profile, practice patterns and outcomes spanning 21 years. Mayo Clin Proc. 2002; 77:429–436.
3. Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet. 2002; 359:2059–2064.
4. Permanyer-Miralda G, Sagristá-Sauleda J, Soler-Soler J. Primary acute pericardial disease: a prospective series of 231 consecutive patients. Am J Cardiol. 1985; 56:623–630.
5. Klein SV, Afridi H, Agarwal D, Coughlin BF, Schielke LH. CT directed diagnostic and therapeutic pericardiocentesis: 8-year experience at a single institution. Emerg Radiol. 2005; 11:353–363.
6. Maggiolini S, Bozzano A, Russo P, et al. Echocardiography-guided pericardiocentesis with probe-mounted needle: report of 53 cases. J Am Soc Echocardiogr. 2001; 14:821–824.
7. Tsang TS, Barnes ME, Hayes SN, et al. Clinical and echocardiographic characteristics of significant pericardial effusions following cardiothoracic surgery and outcomes of echo-guided pericardiocentesis for management: Mayo Clinic experience, 1979-1998. Chest. 1999; 116:322–331.
8. Tsang TS, Freeman WK, Barnes ME, Reeder GS, Packer DL, Seward JB. Rescue echocardiographically guided pericardiocentesis for cardiac perforation complicating catheter-based procedures. The Mayo Clinic experience. J Am Coll Cardiol. 1998; 32:1345–1350.
9. Winter M, Lim I, Joseph M. The disappearing pericardial effusion: a pericardial-pleural fistula. J Am Soc Echocardiogr. 2009; 22:973.e5–973.e7.
10. Reuter H, Burgess L, van Vuuren W, Doubell A. Diagnosing tuberculous pericarditis. QJM. 2006; 99:827–839.
11. Ziskind AA, Pearce AC, Lemmon CC, et al. Percutaneous balloon pericardiotomy for the treatment of cardiac tamponade and large pericardial effusions: description of technique and report of the first 50 cases. J Am Coll Cardiol. 1993; 21:1–5.
12. El Haddad D, Iliescu C, Yusuf SW, et al. Outcomes of cancer patients undergoing percutaneous pericardiocentesis for pericardial effusion. J Am Coll Cardiol. 2015; 66:1119–1128.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download