Abstract
Background and Objectives
This study aims to compare the characteristics, effectiveness and results of transcatheter closure of atrial septal defect between children, adolescents, and adults.
Subjects and Methods
In this study, 683 patients who underwent atrial septal defect closure in the last 10 years were divided into three groups: children (age <12), adolescents (age 12 to 16), and adults (age >16) as group 1, group 2 and group 3, respectively.
Results
The average defect size and incidence of complex atrial septal defect were higher in group 3 (p=0.0001 and 0.03 respectively). While the average size of the devic was higher in adults (22.6±6.4 mm vs. 18.5±4.9 mm; p=0.0001), the ratio of the device size/total septum was higher in both children and adolescents (Group 1 and 2). In the child and adolescent groups and patients with only complex atrial septal defect, the use of techniques, other than standard deployment, was similar in all three groups (p=0.86 and 0.41, respectively). The ratio of the residual shunt was similar in all three groups. Major complications were seen in 5 cases (4 cases with migration, and 1 case with dislocation) in group 3 and 1 case (migration) in group 1.
Studies about the transcatheter atrial septal defect (ASD) closure show that the procedure does not only have an excellent efficacy, but it also has the lowest complication rates. But the results of the procedure may be influenced by various factors that include individual characteristics such as patient age and weight as well as morphological characteristics of the defect and other concomitant diseases.1)2) Similarly, a transcatheter ASD closure would have sui generis challenges, complications and results in both a young child and an adult.3)4) This study was aimed at comparing the different characteristics, efficacies and short-, mid- and long-term results of the ASD closure procedure in children, adolescents and adults.
699 patients were involved in this study, and who underwent an ASD closure procedure with a transcatheter method over the period of 10 years between July, 2004 and August, 2014 at the department of pediatric cardiology of Dr. Siyami Ersek Thoracic and Cardiovascular Surgery Teaching and Research Hospital. The patients were divided into 3 groups: being children (<12 years), adolescents (12 to 16 years) and adults (>16 years) (Group 1, Group 2, and Group 3, respectively). The pre-procedural transesophageal echocardiography (TEE) and/or transthoracic (TTE) echocardiography findings, as well as hemodynamic findings, during catheterization were assessed. All the patients had a diagnosis of secundum ASD with right heart dilation and the maximal diameter (non-stretched diameter) of the defect <38 mm, as measured by TTE or TEE.
Patients found to have a large defect (>38 mm), small shunt (amount of pulmonary flow/amount of systemic flow [Qp/Qs]) <1.5:1 except those with recurrent neurological attacks), or pulmonary vascular resistance of >8 Woods units (WU) under 100% O2 assessed during cardiac catheterization were excluded from the study. Additionally, a transcatheter ASD closure procedure was cancelled for those with additional pathologies requiring surgical intervention, such as partial anomalous pulmonary venous return (at least 2 pulmonary veins) and the device size is much bigger than the atrial septum (10 patients in Group 1, 2 patients in group 2 and 4 patients in Group 3); and these patients were referred to surgery. A total transcatheter ASD closure procedure was performed on 683 patients, 375 in Group 1, 85 in Group 2 and 223 in Group 3.
Informed consent was obtained from all studied patients or their parents and the study was approved by institutional review board.
A percutaneous closure was performed with continuous TTE or TEE guidance. The defects were classified into 2 groups, simple and complex defects. The inclusion criteria for complex ASDs were the following: (1) a stretched ASD diameter ≥25 mm with absent anterosuperior (retroaortic) rim or deficiency (<5 mm) of another rim; or (2) a multifenestrated atrial septum; or (3) an aneurysmal septum regardless of the number of defects. The distances of defects from one another were measured for multifenestrated defects.
At the beginning, balloon-sizing was performed using an AGA sizing balloon (AGA Medical Corporation, Golden Valley, MN, USA) for all patients and both the stretched balloon diameter (SBD) and the stop-flow balloon diameter (SFBD) measurements were obtained. Over time, we came to prefer this procedure for patients with more complex defects, especially floppy and thin rims. In patients for whom balloon-sizing could not be performed, device size was selected as 3-4 mm higher than the measured 2-dimensional defect size and as 1-2 mm than the Doppler color flow diameter for the device closure procedure; however, SBD was measured angiographically when the balloon occluded the defect completely in patients for whom balloon-sizing could be performed and devices selected were generally equal to SBD or 1-2 mm larger than SBD. For patients in whom indentation did not develop during the balloon sizing procedure or who had thin and floppy rims and lack of conformation between echocardiographically measured defect size and SBDs, we preferred to use SFBD measured - both echocardiographically and angiographically - as reference for device selection. It was required to oversize the device in patients with large defects, who had relatively deficient rims and/or thin and floppy rims (oversizing of the device was needed). For multifenestrated ASDs, the defects were closed using a single atrial septal occluder (ASO) using an oversized device, if necessary, in cases where the defects were spaced less than 7 mm from one another. In cases where the defects were located at 7 mm or longer distances from one another and the other defects were hemodynamically important, a second or third ASO or patent foramen ovale (PFO) occluder was used. Additionally, 2 defects were combined in a patient with three defects via septoplasty with a 20-mm diameter Tyshak II balloon (Numed Inc., Hopkinton, NY, USA) and total occlusion was achieved with a single 20-mm device.
Seven different occluder systems were used: Cardi-O-Fix ASD occluder (Starway Medical Technology Incorporation, Beijing, China), Amplatzer Septal Occluder (AGA Medical Corporation, Golden Valley, MN, USA), Cocoon septal occluder (Vascular Innovations Co., Nonthaburi, Thailand), and other septal occluder systems (Cera septal occluder [Lifetech Scientific Corporation, Shenzhen, China], Cardia—atriasept [Cardia Inc., Burnsville, MN, USA], Figulla [Occlutech GmbH., Jena, Germany], and Gore ASD occluder [W.L. Gore & Associates, Flagstaff, AZ, USA]).
The vast majority of devices were deployed using the standard technique. The other techniques were used for patients for whom device implantation with standard deployment could not be performed including rapid deployment, right upper pulmonary vein opening, pushing dilator, using a Hausdorf sheath (Cook Inc., Bloomington, IN, USA), using a Fustar steerable introducer (Lifetech Scientific Corporation, Shenzhen, China), balloon assisted, etc. and a part of these techniques were combinations of some of them.
Apart from patients with deficient or floppy rims, the position and stability of the device were checked via Minnesota maneuver.
Procedural success was defined as stable and proper placement of the occluder to the defect.
Residual shunt after releasing from the device was checked by echocardiography, and was graded as mild, moderate or severe.
Aspirin and antibiotics were given 12 hours and 1 hour before the procedure, respectively. All the patients were prescribed aspirin for 6 months after the procedure. The patients were followed up at day 1, month 1, month 6 and year 1 following the procedure and at intervals of 1 year in the aftermath using TTE-also performing TEE where necessary.
Complications requiring re-intervention, hospitalization, and causing a permanent deficit in the 12-month follow-up period were defined as major adverse events. Besides, other complications, including significant residual leak, significant valve regurgitation, residual ASD >5 mm within the 12-month follow-up period, treatable arrhythmias during the procedure or during the 12-month follow-up period, and an embolized device, or an unsuitable device replaced with an alternate size during a procedure, were defined as minor adverse events.
All the analyses were performed using SPSS version 15.0 for Windows (SPSS Inc., Chicago, IL, USA). The Shapiro–Wilk test was used for the analysis of compliance with the normal distribution. Categorical variables were expressed as absolute numbers or percentage and were compared using chi-square test or Fisher's Exact test, whereas the continuous variables were summarized by mean value±standard deviations. Due to abnormal distributions were observed in all data of each group, the Kruskal-Wallis test was used for comparison of groups, and if a statistically significant difference was found, then comparison of groups was confirmed by the Mann-Whitney U-test. All statistical tests were two-sided. A p<0.05 was considered statistically significant except in comparison with the Mann-Whitney U-test. In this test, as there are three different groups, p<0.017 is considered as statistically significant. Comparisons of the subgroups were done as follows: P1, group 1 vs. group 2; P2, group 1 vs. group 3; P3, group 2 vs. group 3.
The demographic, echocardiographic, cardiac catheterization findings and procedure-related characteristics of the 3 groups undergoing a transcatheter ASD closure are listed in Table 1.
The youngest patient for whom the procedure performed was a 1.5-year-old, weighing 8-kg with growth retardation. While the number of patients at or above the age of sixty was 6, the oldest patient was a 73-year-old female patient who was described as having stage 3 dyspnea, according to New York Heart Association (NYHA), and had a defect of 22.4 mm.
Considering the defect characteristics and hemodynamic findings, the average defect size measured at TTE or TEE, and the complex atrial septal defect rate were higher in Group 3 at a statistically significant level (p=0.0001 and 0.03, respectively). While no differences were detected between the 3 groups in terms of average Qp/Qs rate and average pulmonary artery pressure (p=0.052 and 0.57, respectively), the average right ventricle end-diastolic diameter index was found to be statistically significantly higher in Group 1 (p=0.0001) (Table 1).
Cardi-O-Fix ASD occluders were used in 432 patients, Amplatzer septal occluder in 143 patients, Cocoon septal occluders in 51 patients, Cera septal occluders in 28 patients and Cardia—atriasept, Figulla and Gore ASD occluders in the remaining 29 patients at decreasing numbers. With respect to the distribution of the types of septal occluders used, Cardi-O-Fix septal occluders were used in Group 1, 2 and 3 at the rates of 65%, 56% and 64.6% (p: 0.26), Amplatzer septal occluders at 19.2%, 14.1% and 26.4% (p: 0.028), and other devices (Cocoon septal occluders, Cera septal occluders, Cardia-atrioseptal and others) at 16.5%, 29.4% and 9% (p=0.0001), respectively.
Not only in all cases but also in the ones with complex defects, TTE guidance was prominent in Group 1 whereas TEE guidance was preferred more in groups 2 and 3 (Table 1, p=0.0001, p=0.0001, respectively). The average device size used in adults was larger than the ones in children and in adolescents (22.6±6.4 mm, 18.5±4.9 mm and 20.1±5.7 mm, respectively; p: 0.0001) whereas the average device size/total septum ratio was higher in Group 1 (Table 1). In relation to the average device/defect size ratios, no significant differences were found between three groups (Table 1).
The use of different techniques other than standard deployment was at approximately similar rates in all three groups (p: 0.86). Use of different techniques, other than standard deployment techniques in patients with complex defects were higher in group 1 and group 2 as a comparison to group 3 but the difference was not statistically significant. (Table 1; p=0.41). The youngest patient for whom a different technique was used was a 1.5-year-old, 8-kg patient with growth-development retardation as explained above. In this patient, the 2 dimentional-defect diameter was 15 mm, color flow diameter was 16 mm and total septum diameter was 32 mm. The patient's defect was closed using a 17-mm device and then opening the left atrial disc at the upper left pulmonary vein with dilator assistance (since standard deployment failed).
A multifenestrated defect was present in 63 patients in Group 1 (16.8%), in 5 patients Group 2 (5.9%) and in 27 patients in Group 3 (12.1%) (p=0.02). Due to multifenestrated ASDs, double devices were implanted in the same session in 2 patients, a PFO occluder implanted in 1 patient in group 1. In Group 3, double devices were implanted in the same session in one and triple devices were implanted in another patient in two sessions (Fig. 1).
No statistically significant differences were identified between three groups with respect to floppy or thin rims or use of oversized devices for the closure of other defect(s) accompanying multifenestrated defects (10 patients [2.7%], 1 patient [1.2%] and 3 patients (1.3%) in Group 1, 2 and 3, respectively; p=0.587).
A balloon septoplasty was performed on a 10-year-old female patient in Group 1, who had an aneurysmatic septum with 3 defects, and total occlusion was achieved with a single device. The widest ASD diameter in TEE for this patient was 9 mm and additional defect diameters were 2.4 and 2.1 mm, the total septum diameter was 36 mm and the rims were sufficient (Fig. 2). The stretched diameter, at balloon-sizing performed at the widest defect, was measured as 15 mm and it was seen that the shunt in the defect in the middle disappeared in the meantime, however, the shunt continued through the third defect on the inferior side. The third defect was at a distance of 12 mm to the first defect. Following balloon-sizing, it was considered that the defect on the top and the defect in the middle could be joined and the defect at the bottom could be covered using the device skirt; septoplasty was performed with a 20x4 mm Tyshak 2 balloon. After the defects at the top and the bottom were joined with septoplasty, the second stretch diameter was measured as 20 mm and the defect was closed with a 20 mm ASO. Following closure, it was seen that the third defect remained under the device skirt and there was no residual shunt.
In Group 2, a 14-year-old patient received an ASO home-made fenestration due to pulmonary hypertension. In this patient, the defect diameter at TTE was 19 mm, color flow diameter was 25 mm, pulmonary artery pressure was 54/11 (average: 37) mmHg, Qp/Qs: 1.5 L/min, and pulmonary vascular resistance was 5.1 WU/m2. At the end of vasoreactivity test, these parameters were 43/12 (average: 29) mmHg, 2.7 L/min, and 2.1 WU/m2, respectively. In this patient, the SBD was measured as 26 mm and the defect was closed with a 4-mm, home-made fenestrated device.
In 2 patients in Group 2 who had systemic venous return abnormality (interrupted inferior vena cava [IVC] with azygos continuation to superior vena cava), the closure procedure was successfully completed by means of the right internal jugular vein.
There was a prominent Chiari's network in 5 patients in Group 1, 2 patients in Group 2 and in 3 patients in Group 2. The device implantation was performed successfully in these patients in all groups.
In the same session, a combined procedure was performed in 9 patients in Group 1 (in association with ASD closure coil occlusion of patent ductus arteriosus [PDA] in 5 patients and pulmonary balloon valvuloplasty [PBV] in 4 patients were performed). There were no patients undergoing a combined procedure in other groups.
There were no statistically significant differences between their procedural success rates (99.7%, 100% and 98.7% in Group 1, 2 and 3 respectively, p=0.18). Also the procedural time and fluoroscopy time were similar in three groups (Table 1).
The average follow-up time was similar for all groups: 43, 38 and 47 months for Groups 1, 2 and 3, respectively (Table 2; p=0.56).
In our study, no deaths in early or late stages, aortic erosions, ruptures, thromboembolic events or infective endocarditis were present.
Immediately after procedure, the residual shunt ratio was 14.9%, 7.1% and 14.3% in group 1, group 2 and group 3 respectively (p=0.156). In the aspect of residual shunt, before discharge and during later follow-ups, no statistically significant differences were also detected between three groups (Table 2). Similarly, there were no statistically significant differences between the residual shunt ratio in any of the 3 groups of patients with complex defects, not only immediately after the procedure and evaluation before discharge, but also during all follow-up periods.
Considering all cases, including complex cases, there were no statistically significant differences between groups in terms of severe, moderate, and mild residual ratio. Residual shunt ratios for severe, moderate and mild lesions for group 1, group 2 and group 3 were as follows respectively; 10.7%, 16.6% and 9.3% (p=0.74), 16.1%, 16.6% and 24.8% (p=0.82) and 73.2%, 66.8% and 65.9% (p=0.81)
During the follow-up both severe and moderate degree residual shunts detected immediately after the procedure either decreased or disappeared in all 3 groups. But a mild residual shunt detected immediately after the procedure in a patient who was not seen in regular follow up in group 3, progressed to a severe degree because of device dislocation.
While there were no major complications in Group 2, on the other hand major complication were observed in one patient in group 1 and in 5 patients in group 3 (Table 2; p=0.017). Regarding major complications, migrations were detected in 4 cases and dislocation during follow-up in 1 case in the group of adults. The device was surgically removed in all cases that developed migration. The 40-year-old female case that developed dislocation had a defect size of 27.5 mm, SBD 29.6 mm, stop-flow SBD 28 mm according to TEE and she did not have any rims smaller than 5 mm. This patient received a 30-mm device implantation and no problems apart from a slight residual shunt were identified in the initial controls in the 6th month; however, she did not arrive for the control visits thereafter and she presented 7 years later due to palpitation and slight shortness of breath, which had existed for 2 months. In her TTE examination, it was observed that the implanted device had dislocated on the aortic side and a significant left to right shunt through that site was seen. During her surgery, dislocation of the device at the aortic side was confirmed and it was seen that the parts of the device that were not dislocated were rather well-endothelialized. The patient had no complaints in her consequent follow-ups after removal of the dislocated device and surgical closure of the defect.
Only one major complication was encountered in Group 1. The device was loosened from the delivery wire during recurrent implantation procedure and it embolized into the right atrium (RA). In this 10-year-old male patient, the defect diameter was 20 mm, aortic rim was deficient and other rims were adequate according to TEE findings. Since this patient had an SBD of 23 mm and a TEE defect diameter of 20 mm, a 22-mm device was selected for the occlusion procedure. The embolized device in this patient was removed with the aid of an Amplatz gooseneck snare (eV3 Endovascular Inc., Plymouth, MN, USA) and a 5 French bioptome (Cook Medical Inc., Bloomington, IN, USA); however, the patient was referred to surgery considering that the defect had become wider during recurrent implantation attempts.
Considering the rates at which major complications developed as per the type of septal occluder used, it was seen that 3 out of the 6 total major complications developed during the use of Amplatzer septal occluder (all 3 cases were adults) and the other 3 developed during the use of Cardi-O-Fix septal occluder (2 adult cases, 1 child case).
In terms of minor complications, they were identified as higher in Group 1 as compared to Groups 2 and 3, but this difference was not statistically significant (Table 2; p=0.61).
Regarding the minor complications in Group 1, the embolized device was removed in 2 cases with the transcatheter method (aorta in 1 case, left atrium in 1 case) and another device was successfully implanted in these cases. A complete atrioventricular block (AVB) developed in one case a few hours after the procedure. This case was a 4.5-year-old girl with multiple defects. According to TEE examination, the major defect size was 18.6 mm and there was a minor defect two mm in size, with in the 4mm distance from the site of major defect, total septum length 37 mm, aortic rim was deficient, the other rims were adequate, yet the IVC rim was floppy. In this patient, SBD was measured at 25 mm, SFBD was at 22.5 mm and the standard deployment failed; therefore, the defect was closed by opening the device at the right upper pulmonary vein. Both defects of this patient were closed with a single device and steroids were given for complete an AVB. The rhythm was restored on day 5 following the procedure.
In another case, dislocation and consequent slight residual shunt developed following device implantation. The patient who had a slight dislocation and was not considered to have significant residual shunt was included in the follow-up. Since a slight residual shunt continued in the consequent follow-ups, an invasive hemodynamic assessment was performed 5 years later and the Qp/Qs 1.2 L/min. was detected; therefore, the decision was taken to continue the follow-ups.
The device was embolized to the aorta in one patient in group 2; this embolized device was removed out by a transcatheter and a bigger device was successfully implanted in the same session.
While the device in all embolized cases in Group 3 were surgically removed, one case had slight pericardial effusion and one case developed atrial fibrillation and hypotension during balloon-sizing resulting in some minor complications.
Regarding the major complication rates in cases with complex defects in the three groups, no major complications were seen in the cases with complex defects in Groups 1and 2, while there were two cases of embolizations in which the embolized devices were surgically extracted out in Group 3. Minor complications in Group 1 were at a higher rate as compared to cases with complex defects in Group 2 and 3; however, this difference was not statistically significant (8 cases [7.5%], 1 case [5.5%]and 2 cases [2.5%], respectively; p: 0.27).
Transcatheter closure of secundum ASDs in both children and adults with the presence of an appropriate rim and defect sizes has become almost standard treatment. Even though children seem to be smaller models of adults, there may be difficulties and associated complications in both groups in addition to the facilitation provided by age and large or small size.
As a matter of fact, using a device at a diameter close to the total septum size may have its sui generis challenges and complications during the implantation procedure in a small child with low weight and a large defect.5)6)
However, adults may have a higher rate of large defects, hence complex defects, which may cause failure of a defect closure using a standard technique, and it may be necessary to use a different technique.7) The higher the age, the wider the defect and, the higher the pulmonary hypertension frequency may be encountered.8) Additionally, more elderly patients may present with various types of problems, such as high left atrial pressure due to accompanying rhythm disturbances or reduced left ventricle compliance independently from the defect diameter.9)
While the transcatheter ASD closure age in the literature is reported to be as low as the neonatal period,10) the youngest patient receiving transcatheter ASD closure procedure in this study was a 1.5-year-old, 8kg patient with growth retardation. The total septum diameter of this patient was 32 mm and the patient's defect was closed using a 17mm ASO, which covered almost the entire septum. Since device implantation failed using the standard deployment method, the defect was closed via the dilator-assisted opening of the left atrial disk at the upper left pulmonary vein.
The oldest patient in the study was a 73-year-old female patient who described as having grade 3 dyspnea according to NYHA and had a defect of 22.4 mm. This patient developed atrial fibrillation and hypotension during balloon-sizing and the defect was closed using standard deployment.
Considering the defect characteristics and hemodynamic findings in the study, the average defect diameter and complex atrial septal defect rate measured at TTE or TEE were higher in adults (Group 3) at a statistically significant rate. While the use of techniques other than standard deployment was slightly higher in the group of children, this difference was not statistically significant. Considering the patients with complex defects in three groups, the use of techniques other than standard deployment was at a higher rate in the group of adolescents; however, it was not found to be statistically significant. With regards to the average QP/Qs values and average pulmonary artery pressures, no statistically significant differences were identified between the 3 groups and there were also no elderly patients identified to have high left atrium pressure following the defect closure. On the other hand, the defect in a 14-year-old patient with a high pulmonary artery pressure was closed following home-made fenestration of the device. Unfortunately, no significant decreases were identified in the pulmonary artery pressure following closure, either.
The longer the total septum length is, the longer the diameter of the device used in parallel with the defect size will. The larger total septum diameter in adults as compared to children and adolescents provides the possibility to close wider defects or it may enable the use of larger devices if the defect is complex even if it is not very wide.11)12) Naturally, the average device diameter used in the group of adults is higher than in the group of children and adolescents. On the other hand, it was identified that the average device diameter/total septum ratio in the group of children was statistically significantly higher than in the group of adults. Furthermore, the average device diameter/total septum ratio in both Group 1 and 2 were higher than in the group of adults considering the patients with complex defects in all three groups although the difference was not statistically significant. For that matter, it could be argued that relatively larger devices were actually used in the group of children and adolescents as per the total septum size.
Accompanying associated lesions, such as a lack of continuity of IVC-RA or significant Chiari's network, may also emerge as other reasons that render the entire procedure or only the device implantation difficult.13)
Since 2 cases in Group 1 did not have IVC-RA continuity, the defects were successfully occluded by means of the right internal jugular vein. Although there are patients with significant Chiari's networks in three groups, there were no patients causing significant difficulties during implantation.
In patients with multiple defects who are indicated and have eligible total septum lengths and rims, cribriform ASO or PFO occluders may be used or multiple device implantations may be performed. On the other hand, additional defect(s) that is/are not hemodynamically important may not be closed if it is not clinically required or if the remaining defect(s) may potentially close automatically in the future.14) While a PFO occluder was implanted in 1 case and double ASO implantation was made in 2 cases during the same session in Group 1, double ASO implantation was made in 2 cases during the same session in Group 3. Since a hemodynamically significant shunt from the third defect was detected in a case that received double ASO implantation during the same session in Group 3, the third ASO was successfully implanted.
We are convinced that joining the defects using balloon septoplasty may prevent the use of multiple devices, if the total septum is not eligible for implantation of multiple devices or if there is concern that there will be a too high metal load in the heart, even if it is eligible in patients with multiple defects. Balloon septoplasty was performed in this study on a 10-year-old female patient with a total septum length of 36 mm, an aneurysmatic septum having 3 defects and total occlusion was ensured with a single 20-mm device. If there is no residual shunt from the adjacent defects during balloon sizing of major defects, these multiple defects could be closed with one device for example cribriform ASD device or Gore septal occluder. Even an ordinary ASD device may work well to close multiple defects. We preferred balloon septoplasty since there was still residual shunt from the 3rd defect during balloon sizing and the defect that placed between the other two defects was small. However, it must be considered that while combining defects with balloon septoplasty the newly formed defect could be so large that could not be closed by device.
Other congenital heart diseases accompanying ASD causing symptoms and signs result in an earlier cardiologic assessment; therefore, diagnosis of ASD with accompanying abnormalities is made at younger ages as compared to isolated ASD. For that reason, the possibility to perform a combined procedure in the same session would naturally be higher in patients undergoing a transcatheter ASD closure in the pediatric age group.15) There were no patients undergoing a combined procedure during the same session in the group of adults while a total of 9 patients in Group 1 underwent transcatheter PDA closure or PBV procedure in the same session.
It has been reported that TTE guidance during a transcatheter ASD closure in children and some adults is safe and effective even in complex ASD patients with good acoustic windows, significantly reducing the procedure time and also providing increased patient comfort.16)
Indeed, the closure rates of a transthoracic echocardiography were found to be higher in the group of children and adolescents in comparison with adults since their acoustic characteristics were more appropriate. While TEE guidance was preferred more often than TTE during closure procedures in patients in all three groups with complex defects, TTE guidance was still at higher rates in children with complex defects in comparison with adolescents and adults.
Residual shunt rates immediately after the procedure and in early follow-up periods were reported to be higher; however, the residual shunt rates at the end of the follow-up were reported at rates of 3.8-3.9% in adult cases with transcatheter ASD closure and at rates of 2.5-5.3% in children and adolescent cases. This rate may naturally be as high as 28-29% in complex cases, for example, cases with multiple defects.11) In this study, there were no statistically significant differences between the three groups regarding the residual shunt rates immediately after the implant, before discharge, and at later follow-ups with respect to not only all the patients but also all patients with complex defects.
Normally, procedure-related complication development risk is low.17) On the other hand, the incidence rates of major or minor complications may be expected to increase in children are inversely proportional with age, while in adults it is parallel and directly proportional with age or complication level of defects. However, El Said et al. reported no differences in patients below the age of 3, patients under 15 kg, or patients with multiple defects in terms of the development rate of adverse events as compared to other patients.18) Similarly, Bisnoi et al. did not report any significant complications in their study conducted on infants below 8 kg, either.3) Diab et al.,19) on the other hand, reported a major complication in only 1 of their studies, which was conducted on 15 infants below the age of 1. However, they found that the incidence rate of minor complications in their own study was higher than the minor complication rates reported for children or adults in other studies.Given that the atrial septum size would be wider in adults as compared to children, it is normal that the defects to be closed transcatheterally are wider. The use of larger devices due to wider defects may translate into more complications. On the other hand, the rims around the wider defect in adults would also be larger in comparison with children; therefore, the wide defects in such cases may be successfully closed. On the other hand, if the wide defect is accompanied by the deficiency or absence of an anterior rim or deficiency of a posterior or atrial septal misalignment, which would cause the wide defect to be termed a complex defect, different complications due to the use of techniques other than the standard technique or an oversized device in such a case.12)
Another reason for concern is whether the use of multiple devices increases the risk of complication or not.20) While the multiple device implantation has been performed in most patients, children or adult, in a successful way without significant complications,21) it has been reported that multiple device implantation in the atrial septum of some patients may prevent the conformation of the device to the septum, which may result in friction and erosion in the atrial wall.22)
Device embolization and dislocation are the most important complications that may be classified as major or minor complications. Device embolization/dislocation, which has been reported to develop at rates of 0.5-1% and generally during or following the procedure, has been associated with defect size, device size, use of a device smaller than the defect, deficient and thin rims, device mobility following implantation or over-performance of the Minnesota maneuver.23) Furthermore, dislocation and embolization before endothelialization following the initial discharge have been reported to be due to a Valsalva maneuver such as lifting a heavy object, which suddenly increases the afterload and reduces the fill level of the right ventricle.24)
In our cases, a major complication was not observed in the adolescent group. Major complications were identified at a statistically significantly higher rate in Group 3 in comparison with Group 1, and minor complications were identified to be slightly higher in Group 1 compared to Groups 2 and 3, although this difference was not significant.
With regards the major and minor complication rates in cases with complex defects, no major complications were seen in cases with complex defects in Group 1and 2 while 2 cases in Group 3 had device embolization, hence referred to surgery.
As for minor complications, they were at a higher rate in cases with complex defects in Group 1; however, this difference was not statistically significant.
The retrieval success of embolized devices has been reported at around 50-75% in the literature; furthermore, it was stated that the most appropriate locations for retrieving the device after it has been embolized is the pulmonary artery and aorta.25)
The devices in all cases developing migration in the group of adults in this study were surgically explanted. The reason behind the preference for surgery in these patients was the fact that the level of experience was low in initial years in 1 patient, device embolization was noticed the date after the procedure in 1 patient and the wide defect in this patient who received a 36 mm-device; defect diameters were also considered to be iatrogenically widened in two patients due to recurrent implantation procedures and it was not possible at the time to find a larger device with an appropriate diameter.
The devices in all 3 patients with device embolization in Group 1 (aortic arch in 1 patient, left atrium in 1 patient and right atrium in the other one) were removed using the transcatheter method. While the defect was closed in 2 patients with a different device, it was considered that the defect was widened and the total septum length would not be enough during recurrent implantation attempts in 1 patient, hence the device was transcatheterally removed and the patient was referred to surgery under elective conditions.
Also in group 2 in one patient the embolized device was removed out by transcatheter way and a bigger device was successfully implanted.
Device dislocation is a rare complication that may develop during, immediately after the procedure or in the late period and may require surgery.26) Dislocation was identified in this study in 2 cases, each in Group 1 and 3. It was identified in the 5-year-old case after the unscreening of the device and in the 40-year-old case, who failed to regularly come for follow-up visits, probably 7 years after the procedure although the date is not exactly certain. While only follow-up was enough for the first case, surgical intervention became a necessity in the second case.
There may be various tachyarrythmias following transcatheter ASD closure while there may also be various rhythm problems in the form of tachycardia or AVB following its closure or in the aftermath. Non-sustained supraventricular tachycardias (SVT) are rather frequent in the early period while sustained atrial fibrillation, flutter, and other SVTs are more rare. Similarly, 1st, 2nd and 3rd degree blocks, most of which improve in the first week may be seen, while blocks developing in the late period have also been reported.27)28)
With respect to important rhythm problems, post-procedural complete AVB developed in 1 case in Group 1. While there was no rhythm problem in the adolescent group, in the adult group, one elderly patient developed atrial fibrillation during balloon-sizing; therefore, a balloon-sizing procedure could not be performed in this patient.
The device selected for the 4.5-year-old case that developed complete AVB due to the wide defect, deficient aortic rim and floppy IVC rim, was at a size that was almost close to the total septum size including the device skirt. According to the literature review, cases have been reported whose block healed spontaneously within hours, days or a couple weeks with medical treatment or via the replacement of the device with a smaller device or its surgical removal.29) On the contrary, a case that received permanent pacemaker implantation despite medical treatment and even retrieval of the device has also been reported.30) Similarly, a case with 2nd degree AVB following transcatheter ASD closure had an initial regression in 2. degree AVB with steroid treatment; however, the AVB progressed in the consequent years with the development of complete AVB in year 4 requiring permanent pacemaker implantation.28) Our case in which the patient developed AVB a few hours following transcatheter ASD closure, steroid treatment was provided and it was planned to expand the device if AVB would not improve within 1 week with treatment and the rhythm was restored on Day 5 following the procedure in this patient.
Firstly, a clear decision could not be made on the maximum age limit in Group 1 or Group 2 during the creation of groups and those above the age of 16 were included in the adult group in line with literature. However, patients at younger ages could also be in the adult group depending on their body weight and height. Secondly, literature was strictly followed also in terms of the complex defect classification. On the other hand, for young children with a stretched ASD diameter < 25 mm and deficiency or absence of certain rims, certain defects could also be classified as complex defects depending on the child size or total septum length. Finally, this is a retrospective study spanning a period of 10 years. Another limitation regarding follow-up results is that certain cases did not come back for regular control visits or they only came in cases of complaints.
Nowadays a transcatheter ASD closure is a routine procedure performed in secundum type ASD's. With the increased experience and technological developments the procedure age has become as low as the infantile period in compulsory situations. However, it may have unique challenges and possible complications, which may result from patient features or defect anatomy, not only in children and adolescents, but also in adults.
The most important point to be kept in mind in order to avoid challenges during the procedure and major complications is to conduct a detailed evaluation of the patient before the procedure. Nevertheless serious complications may also occur in the late period.
Figures and Tables
Fig. 1
Three atrial septal occluders in the same patient. Thin and thick long arrows show the first 2 devices previously implanted by sandwich method, the short arrow indicates proximal end of the third device implanted in a different session.
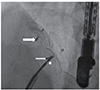
Fig. 2
Aneurysmatic septum and three defects at bicaval position on transesophageal echocardiography in a 10-year-old female patient performed balloon septoplasty.
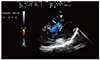
Table 1
Patients, defect characteristics, and procedural features

Values are presented as mean±standard deviation or number (%). p, p1, p2, p3: Comparison of all groups, group 1 and 2, group 1 and 3 and group 2 and 3, respectively. *Statistically significant, †Fisher's Exact Test was used. RVEDd: right ventricle end-diastolic diameter, TTE: transthoracic echocardiography, TEE: transesophageal echocardiography, mm: millimeter, min: minute
Table 2
Residual shunts and complications, and follow-up results

References
1. Kim NK, Park SJ, Choi JY. Transcatheter closure of atrial septal defect: does age matter? Korean Circ J. 2011; 41:633–638.
2. Park SJ, Kim NK, Kim JO, Yoo BW, Choi JY, Sul JH. Morphologic Characteristics and RelatingFactors to the Need of Technical Modification in Transcatheter Closure of Large Atrial Septal Defect (>/=25 mm). Korean Circ J. 2010; 40:191–196.
3. Bishnoi RN, Everett AD, Ringel RE, et al. Device closure of secundum atrial septal defects in infants weighing less than 8 kg. Pediatr Cardiol. 2014; 35:1124–1131.
4. Lairakdomrong K, Srimahachota S, Lertsapcharoen P, Chaipromprasit J, Boonyaratavej S, Kaewsukkho P. Clinical results of large secundum atrial septal defect closure in adult using percutaneous transcatheter Cocoon atrial septal occluder. J Med Assoc Thai. 2013; 96:1127–1134.
5. Cardenas L, Panzer J, Boshoff D, Malekzadeh-Milani S, Ovaert C. Transcatheter closure of secundum atrial defect in small children. Catheter Cardiovasc Interv. 2007; 69:447–452.
6. Dalvi B, Pinto R, Gupta A. Device closure of large atrial septal defects requiring devices > or =20 mm in small children weighing <20 kg. Catheter Cardiovasc Interv. 2008; 71:679–686.
7. Zhang H, Chen Q, Chen LW, Cao H, Zhang GC, Chen DZ. Intraoperative device closure of atrial septal defects in the older population. J Cardiothorac Surg. 2011; 6:123.
8. Gabriels C, De Meester P, Pasquet A, et al. A different view on predictors of pulmonary hypertension in secundum atrial septal defect. Int J Cardiol. 2014; 176:833–840.
9. Lakhdhar R, Drissa M, Drissa H. Natural history of atrial septal defect in the sixth decade : study of 5 cases. Tunis Med. 2013; 91:243–247.
10. Beitzke A, Zobel G, Nagel B, Koestenberger M. Transcatheter closure of an atrial septal defect in a newborn with aortic stenosis. Acta Paediatr. 2009; 98:582–583.
11. Du ZD, Hijazi ZM, Kleinman CS, Silverman NH, Larntz K. Amplatzer Investigators. Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: results of a multicenter nonrandomized trial. J Am Coll Cardiol. 2002; 39:1836–1844.
12. Knirsch W, Dodge-Khatami A, Valsangiacomo-Buechel E, Weiss M, Berger F. Challenges encountered during closure of atrial septal defects. Pediatr Cardiol. 2005; 26:147–153.
13. Cooke JC, Gelman JS, Harper RW. Chiari network entanglement and herniation into the left atrium by an atrial septal defect occluder device. J Am Soc Echocardiogr. 1999; 12:601–603.
14. Masseli J, Bertog S, Stanczak L, et al. Transcatheter closure of multiple interatrial communications. Catheter Cardiovasc Interv. 2013; 81:825–836.
15. Turkay S, Abdullah E, Celal A, et al. Multiple transcatheter interventions in the same session in congenital cardiopathies. J Cardiovasc Dis Res. 2010; 1:181–190.
16. Erdem A, Sarıtas T, Zeybek C, et al. Transthoracic echocardiographic guidance during transcatheter closure of atrial septal defects in children and adults. Int J Cardiovasc Imaging. 2013; 29:53–61.
17. Geva T, Martins JD, Wald RM. Atrial septal defects. Lancet. 2014; 383:1921–1932.
18. El-Said H, Hegde S, Foerster S, et al. Device therapy for atrial septal defects in a multicenter cohort: acute outcomes and adverse events. Catheter Cardiovasc Interv. 2015; 85:227–233.
19. Diab KA, Cao QL, Bacha EA, Hijazi ZM. Device closure of atrial septal defects with the Amplatzer septal occluder: safety and outcome in infants. J Thorac Cardiovasc Surg. 2007; 134:960–966.
20. Mahadevan VS, Gomperts N, Haberer K, et al. Transcatheter closure of atrial septal defects with multiple devices in adults: procedural and clinical outcomes. Int J Cardiol. 2009; 133:359–363.
21. Butera G, Romagnoli E, Saliba Z, et al. Percutaneous closure of multiple defects of the atrial septum: procedural results and long-term follow-up. Catheter Cardiovasc Interv. 2010; 76:121–128.
22. Awad SM, Garay FF, Cao QL, Hijazi ZM. Multiple Amplatzer septal occluder devices for multiple atrial communications: immediate and long-term follow-up results. Catheter Cardiovasc Interv. 2007; 70:265–273.
23. Kawamura A, Nishiyama N, Kawakami T, Fukuda K. Retrieval of embolized Amplatzer septal occluder using ablation catheter and triple-loop snare. Cardiovasc Interv Ther. 2014; 29:350–353.
24. Mashman WE, King SB, Jacobs WC, Ballard WL. Two cases of late embolization of Amplatzer septal occluder devices to the pulmonary artery following closure of secundum atrial septal defects. Catheter Cardiovasc Interv. 2005; 65:588–592.
25. Goel PK, Kapoor A, Batra A, Khanna R. Transcatheter retrieval of embolized AMPLATZER Septal Occluder. Tex Heart Inst J. 2012; 39:653–656.
26. Dhaliwal RS, Singh H, Swami N, Srivastava V. Removal of displaced and impacted ASD device after 4 years. Thorac Cardiovasc Surg. 2009; 57:233–235.
27. Szkutnik M, Lenarczyk A, Kusa J, Białkowski J. Symptomatic tachy- and bradyarrhythmias after transcatheter closure of interatrial communications with Amplatzer devices. Cardiol J. 2008; 15:510–516.
28. Nehgme RA, Huddleston AR, Cheatham JP. Progression to late complete atrioventricular block following amplatzer device closure of atrial septal defect in a child. Pediatr Cardiol. 2009; 30:367–370.
29. Rohit MK, Puri K, Vadivelu R. Reversible complete atrioventricular block after percutaneous ASD device closure in a child <15 kg. Indian Heart J. 2014; 66:366–369.
30. Amoozgar H, Ahmadipoor M, Amirghofran AA. Complete heart block following transcatheter closure of atrial septal defect due to growth of inflammatory tissue. Pediatr Cardiol. 2014; 35:1301–1303.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download