Abstract
Background and Objectives
The relationship of synergy between percutaneous coronary intervention with taxus and cardiac surgery (SYNTAX) score and development of atrial fibrillation (AF) after coronary artery bypass surgery (CABG) has not been studied. Therefore, we assessed the relationship between the SYNTAX score and development of AF after CABG (POAF).
Subjects and Methods
The medical records of consecutive patients, who underwent CABG surgery from January 2013 to September 2015, were retrospectively reviewed for the development of AF in the postoperative period. SYNTAX score, clinical and echocardiographic parameters were evaluated. The independent variables for the development of POAF were defined and their predictive values were measured.
Results
The study group consisted of 106 patients, of which 36 (34%) developed POAF. Age, hypertension, stroke, chronic obstructive pulmonary disease (COPD), heart failure (HF), diabetes mellitus (DM), left atrial diameter, neutrophil/lymphocyte ratio, platelet large cell ratio, creatinine, blood urea nitrogen and SYNTAX score were identified as important variables for the development of POAF. However, in logistic regression analysis COPD (OR=19.313, 95% CI=2.416-154.407, p=0.005), HF (OR=28.362, 95% CI=2.034-395.515, p=0.013), SYNTAX score (OR=0.863, 95% CI=0.757-0.983, p=0.026), and DM (OR=20.770, 95% CI=3.791-113.799, p<0.001) appeared as independent variables predicting the development of POAF. In receiver operation characteristic analysis, SYNTAX score (≥22.25) (AUC=0.777, 95% CI=0.676-0.877, p<0.001) was one of the strongest predictors for the development of POAF.
Atrial fibrillation (AF) is the most common arrhythmia occurring after coronary artery bypass graft (CABG) surgery and is seen in approximately 15-30% of the patients.1) Development of AF after cardiac surgery is associated with increased morbidity, mortality, longer hospital stay and a two to three-fold increase in postoperative stroke.2) Older age, obesity, hypertension (HT), prior AF and congestive heart failure (HF) are associated with higher risk of developing AF after cardiac surgery.3) There is a relationship between AF and coronary artery disease (CAD).4)5)
Synergy between percutaneous coronary intervention (PCI) with taxus and cardiac surgery (SYNTAX) score is the angiographic scoring system and is widely used to evaluate the severity and complexity of CAD. It is used in the estimation of long-term outcomes of CAD and in the selection of the treatment modality. Its efficacy has been demonstrated in various studies.6)7) The aim of this study was to investigate the relationship between the incidence of postoperative atrial fibrillation (POAF) and severity and complexity of CAD.
The study group consisted of 106 consecutive patients, who underwent on-pump CABG surgery from January 2013 to September 2015. The data from patients were retrospectively analyzed for the development of AF in the postoperative period until discharge. The study was approved by the local ethics committee of the university, Faculty of Medicine.
The patients were monitored using a heart rhythm monitor at an intensive care unit. In addition, daily electrocardiographic recordings were obtained during the hospital stay both in the intensive care unit and regular ward. New-onset postoperative AF (as classified by the Society of Thoracic Surgeons) was defined as AF or atrial flutter occurring postoperatively and requiring medical treatment (beta-blocker, calcium channel blocker, amiodarone, anticoagulants, and cardioversion). The patients, who developed AF in the postoperative period until discharge, were included in the POAF group. Patients with paroxysmal or persistent AF, patients who were receiving antiarrhythmic medications, and those who underwent pharmacological or electrical cardioversion before CABG surgery due to reasons other than AF, patients who underwent redo CABG surgery and other cardiac procedures in addition to CABG or who were planned to undergo emergency surgery, and patients who had significant valvular disease or prosthetic valvular disease were excluded from the study. Postoperative period was described as from operation to discharge. Mean postoperative period was 11 days for POAF group, 8 days for without POAF. Preoperative AF has been excluded with serial ECG for several days. Mean follow up duration of serial ECG was seven days.
The patient's data including age, gender, history of HT, chronic kidney disease, diabetes mellitus (DM), HF, chronic obstructive pulmonary disease (COPD), congenital heart disease, valvular heart disease, liver disease, stroke, thyroid disease, preoperative drug use (beta blockers, and statins), and echocardiographic variables such as ejection fraction (EF), left atrial diameter, and presence of valvular disease were retrospectively retrieved from the medical charts and included in the analysis. Heart failure was defined as progressive dyspnea associated with clinical signs of pulmonary congestion and a left ventricular ejection fraction (LVEF) <50%. Laboratory parameters were studied from venous blood sample one day before the surgery.
All patients underwent transthoracic echocardiography using GE Vivid S5 (General Electric Ving Med Systems, Horten, Norway) echocardiography device and Mass S5 probe (2-4 MHz) one day before surgery. Standard two-dimensional and color flow Doppler views were acquired according to the guidelines of American Society of Echocardiography and European Society of Echocardiography.8) The ejection fraction was measured according to Simpson's method. Anteroposterior left atrial diameter in parasternal long axis view was measured using two-dimensional echocardiography at the end of left ventricular systole.
Coronary angiography was performed by the Judkins technique. All lesions causing ≥50% stenosis in a coronary artery with a diameter ≥1.5 mm were included in the SYNTAX score calculation. For calculation, website software (http://www. SYNTAXcore.com) was used. Scoring was performed for each patient in keeping with the following parameters: coronary dominance, number of lesions, segments included per lesion, the presence of total occlusion, bifurcation, trifurcation, aorto-osteal lesion, severe tortuosity, calcification, thrombus, diffuse/small vessel disease, and lesion length >20 mm. SYNTAX score was evaluated separately by 2 interventional cardiologists blinded to the study protocol and patient characteristics. In case of a contradiction between two results, the opinion of a senior interventional cardiologist was applied and a common consensus was obtained.
All descriptive statistics were calculated and shown for each variable. Continuous variables were expressed as mean±standard deviation (median) and categorical variables were expressed as frequency (n) and percentage (%). Shapiro-Wilk test of normality was used to evaluate the distribution of continuous variables. Either Student's t-test or Mann-Whitney U-test was used to compare two groups for continuous variables depending on their distribution characteristics. For categorical variable comparisons, Pearson Chi Square test or Fisher's Exact test was used, where appropriate. To predict POAF, univariate logistic regression analyses were carried out for each predictive variable. Those were found to be significant in univariate hypothesis tests. In order to consider the interactions between these variables and statistical adjustments, a multivariate logistic regression model with this set of predictive variables was also performed as the final step.
Furthermore, receiver operation characteristic (ROC) curve analysis was applied to evaluate the diagnostic performance of vitamin B12 for differentiating low and high SYNTAX score patients. Level of significance was accepted to be 0.05 throughout the study. All statistical analysis was performed with SPSS version 18.0 software package (SPSS Inc., Chicago, IL, USA).
The present study included 106 consecutive patients, of which of 36 (34%) developed POAF. The median time for POAF occurrence was 1.8 days. The mean length of hospital stay was longer in patients who developed POAF (11±2 vs. 1.7±8 days, p<0.001). The main characteristics of patients who developed POAF and those who did not are presented in Table 1. All patients were on beta-blocker and statin therapy. 91.5% of patients were on angiotensin converting enzyme inhibitor/angiotensin receptor blocker therapy (Table 1). Patients that developed POAF were older, and the prevalence of cardiovascular risk factors such as HT, DM, and stroke were higher and COPD was more common in these patients (p<0.05) (Table 1). The SYNTAX score was higher in the POAF group (p<0.001) (Table 1). Laboratory and echocardiography parameters are presented in Table 2. Neutrophil lymphocyte ratios (NLR) and platelet large cell ratios (p-LCR) were significantly higher in patients with POAF (2.9±2.0 vs. 2.1±0.8, and 31.5±6 vs. 27.4±6.9, respectively, all p< 0.05) (Table 2). Additionally, blood urea nitrogen (BUN) and serum creatinine levels were significantly higher in the POAF group (46.3±21.7 vs. 36.9±15.4, and 1.05±0.26 vs. 1.2±1.07, respectively, all p< 0.05) (Table 2). Patients who developed POAF had larger left atrial diameters (39.9±7.7 mm vs. 37.3±5.7 mm, p=0.002) (Table 2). LVEF was significantly lower in patients with POAF (51.5±9.6% vs. 54.2±7.7%, p=0.03) (Table 2).
The parameters that showed a significant difference in univariate analysis were further evaluated using ROC analysis, and cut-off values were determined for these variables in predicting the development of POAF. The cut-off value for left atrial diameter was 36.5 mm (area under the curve [AUC]=0.68, 95% confidence interval [CI]=0.578-0.789), and for age was 58.5 years (AUC=0.625, 95% CI= 0.514-0.735).
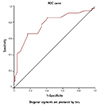
Among these independent variables, SYNTAX score (≥22.3) was found to be one of the strongest predictors (AUC=0.777, 95% CI=0.676-0.877; 86.1% sensitivity, 58.6% specificity) for the development of POAF (Fig. 1). Univariate logistic regression analysis demonstrated that age, COPD, HF, TIA/stroke, DM, and SYNTAX score were significantly associated with development of POAF following CABG (Table 3). In logistic regression analysis, COPD (odds ratio [OR]=19.313, 95% CI=2.416-154.407, p=0.005), HF (OR=28.362, 95% CI=2.034-395.515, p=0.013), and SYNTAX score (OR=0.863, 95% CI=0.757-0.983, p=0.026), DM (OR=20.770, 95% CI=3.791-113.799, p<0.001) appeared as independent variables predicting the development of POAF (Table 4).
AF is a growing global health concern and is linked to a wide range of medical complications including HF, ischemic stroke, and death. It is estimated that AF may account for up to 10% to 15% of all strokes, with an associated mortality of up to 1.9-fold.9)
The present study was the first to evaluate the predictive value of the SYNTAX score in the development of POAF. COPD, HF, DM and higher SYNTAX score were found to be independent variables predicting the development of POAF. In previous studies, advanced age, male gender, chronic HF, preoperative AF attacks, COPD, chronic renal disease, DM and rheumatic heart disease, metabolic syndrome, and obesity were reported to be preoperative clinical parameters predicting the development of POAF.10)11)
COPD is an independent risk factor for arrhythmias, especially AF, and cardiovascular morbidity and mortality. In a large-scale, retrospective, case-control study, patients with COPD had a 4.41 times higher risk of AF (95% CI 4.00-4.87), and COPD is present in 10-15% of patients with AF.12)13) COPD was found to be an important variable predicting the development of postoperative AF in this study. We believe that the relation between COPD and POAF depends on hypoxia, hypercapnia, acidosis, inflammation, electrolyte disturbance, autonomic dysfunction and pulmonary hypertension.
AF is one of the most common comorbidities in patients with HF, while HF is also common in AF patients.14) The prevalence of AF in patients with HF ranges from 15% to 50%.15) AF exerts a negative hemodynamic effect by decreasing cardiac output via the loss of atrial contraction and impaired ventricular rate control. AF is associated with a worse prognosis in HF.16) HF was found to be an important variable predicting the development of postoperative AF in this study.
Diabetes increases the risk of developing AF and especially young patients with DM have a high relative risk.17) A recent meta-analysis of cohort and case control study showed that patients with DM had an approximately 40% greater risk of AF, but this varied from 70% in studies adjusted only for age and sex to 24% in those adjusted for more variables.18) DM was found to be an important variable predicting the development of postoperative AF in this study. The high rate of development of POAF in DM patients is thought to be caused by the inflammatory process of the disease. In our study, diabetic patients were on intensive insulin therapy during hospitalization. There was no difference determined with respect to fasting plasma glucose in two groups. We thought this result was due to strict glucose control through intensive insulin therapy.
P-LCR illustrates the percentage of large platelets. The normal range is usually below 50%. Increased p-LCR level was independently associated with a higher Framingham risk score.19) P-LCR level is an independent prognostic factor in PCI-treated acute myocardial infarction.20) In our study p-LCR level was significantly higher in patients with POAF. We thought this association was closely related to higher p-LCR level and extensivity of CAD.
NLR is a marker of systemic inflammation that has been shown to predict mortality in patients with ischemic heart disease and peripheral vascular disease, and may be useful for predicting adverse cardiovascular events including left ventricular remodelling.21) NLR is a powerful predictor of baseline or postsurgery AF.21) In our study, NLR was significantly higher in patients with POAF.
Chronic renal disease may activate the renin-angiotensin-aldosterone (RAS) system, leading to LV hypertrophy and fibrosis. The incidence of AF was significantly higher in impaired renal function.22) In our study, urea and creatinine levels were significantly higher in patients with POAF.
Lip and Beevers4) described an incidence of 46% and stated that CAD is one of the most common causes of AF in the western world. Kralev et al.5) reported that the incidence of CAD was 34% in patients with AF. Several studies have demonstrated a relationship between CAD and POAF.10)11) The SYNTAX score takes into account anatomic complexities including calcification, total occlusion, bifurcation lesions, thrombus, and long lesions. In spite of some limitations, such as absence of clinical profiles and wide interobserver variation, the score is considered to be a useful predictor for the extent of coronary disease, and provides important information for deciding the revascularization strategy. SYNTAX score, which is used in the evaluation of angiographic severity of coronary lesions, has already been shown to predict mortality.6)7) SYNTAX score predicts short- and long-term adverse events following revascularization.23) Although, SYNTAX score predicts early adverse events in patients undergoing off-pump CABG, there was no significant difference between a higher SYNTAX score and development of POAF.24) Our study design was conducted with on-pump coronary artery bypass graft surgery patients due to a lower incidence of AF in patients undergoing off-pump surgery.25)
The relation between coronary ischemia and AF is well established.8)9) Patients with higher SYNTAX score are known to have more severe coronary ischemia, and also increased short- and long-term adverse events following revascularization. In this study, the SYNTAX score was found to be an independent predictor of the development of POAF. Further studies with larger populations are needed in this field.
Our study has some limitations. First was a retrospective study design. Second was ECG monitoring in a hospital setting without performing a follow-up after discharge diagnosed AF. Third was the small sample size of the present study. Fourth, the preoperative ECG follow up time was too short to detect paroxysmal AF. Although this is the first study investigating the relationship between POAF and the severity and complexity of CAD, further prospective studies with a larger number of patients are required due to the relatively small number of patients in the present study.
Figures and Tables
Fig. 1
ROC curve for SYNTAX score for predicting postoperative atrial fibrillation. ROC: receiver operation characteristic, SYNTAX: synergy between percutaneous coronary intervention with taxus and cardiac surgery.
References
1. Blommaert D, Gonzalez M, Mucumbitsi J, et al. Effective prevention of atrial fibrillation by continuous atrial overdrive pacing after coronary artery bypass surgery. J Am Coll Cardiol. 2000; 35:1411–1415.
2. Amar D, Shi W, Hogue CW Jr, et al. Clinical prediction rule for atrial fibrillation after coronary artery bypass grafting. J Am Coll Cardiol. 2004; 44:1248–1253.
3. American heart association heart disease and stroke statistics update at-A-glance. Circulation. 2007; 115:e69–e171.
4. Lip GY, Beevers DG. ABC of atrial fibrillation. History, epidemiology, and importance of atrial fibrillation. BMJ. 1995; 311:1361–1363.
5. Kralev S, Schneider K, Lang S, Süselbeck T, Borggrefe M. Incidence and severity of coronary artery disease in patients with atrial fibrillation undergoing first-time coronary angiography. PLoS One. 2011; 6:e24964.
6. Van Gelder IC, Groenveld HF, Crijns HJ, et al. Race II Investigators. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010; 362:1363–1373.
7. Crijns HJ, Van Gelder IC, Van Gilst WH, Hillege H, Gosselink AM, Lie KI. Serial antiarrhythmic drug treatment to maintain sinus rhythm after electrical cardioversion for chronic atrial fibrillation or atrial flutter. Am J Cardiol. 1991; 68:335–341.
8. AFFIRM Investigators. Atrial Fibrillation Follow-up Investigation of Rhythm Management. Baseline characteristics of patients with atrial fibrillation: the AFFIRM study. Am Heart J. 2002; 143:991–1001.
9. Sianos G, Morel MA, Kappetein AP, et al. The SYNTAX score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005; 1:219–227.
10. Aksakal E, Tanboğa IH, Kurt M. Predictors of coronary lesions complexity in patients with stable coronary artery disease. Angiology. 2013; 64:304–309.
11. Ucar H, Gur M, Borekci A, et al. Relationship between extent and complexity of coronary artery disease and different left ventricular geometric patterns in patients with coronary artery disease and hypertension. Anatol J Cardiol. 2015; 15:789–794.
12. Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998; 98:946–952.
13. Banach M, Rysz J, Drozdz JA, et al. Risk factors of atrial fibrillation following coronary artery bypass grafting: a preliminary report. Circ J. 2006; 70:438–441.
14. Sidney S, Sorel M, Quesenberry CP Jr, DeLuise C, Lanes S, Eisner MD. COPD and incident cardiovascular disease hospitalizations and mortality: Kaiser Permanente Medical Care Program. Chest. 2005; 128:2068–2075.
15. Caglar IM, Dasli T, Turhan Caglar FN, Teber MK, Ugurlucan M, Ozmen G. Evaluation of atrial conduction features with tissue Doppler imaging in patients with chronic obstructive pulmonary disease. Clin Res Cardiol. 2012; 101:599–606.
16. Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort: The Framingham heart study. JAMA. 1994; 271:840–844.
17. Middlekauff HR, Stevenson WG, Stevenson LW. Prognostic significance of atrial fibrillation in advanced heart failure: A study of 390 patients. Circulation. 1991; 84:40–48.
18. Huxley RR, Filion KB, Konety S, Alonso A. Meta-analysis of cohort and case-control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am J Cardiol. 2011; 108:56–62.
19. Schoen T, Pradhan AD, Albert CM, Conen D. Type 2 diabetes mellitus and risk of incident atrial fibrillation in women. J Am Coll Cardiol. 2012; 60:1421–1428.
20. Rechciński T, Jasińska A, Foryś J, et al. Prognostic value of platelet indices after acute myocardial infarction treated with primary percutaneous coronary intervention. Cardiol J. 2013; 20:491–498.
21. Shao Q, Chen K, Rha SW, Lim HE, Li G, Liu T. Usefulness of Neutrophil/Lymphocyte Ratio as a Predictor of Atrial Fibrillation: A Meta-analysis. Arch Med Res. 2015; 46:199–206.
22. Kwon CH, Kim J, Kim MS, et al. Impact of Impaired Renal Function on the Incidence of Atrial Fibrillation following Radiofrequency Ablation of Cavotricuspid Isthmus-Dependent Atrial Flutter. Korean Circ J. 2015; 45:473–478.
23. Valgimigli M, Serruys PW, Tsuchida K, et al. Cyphering the complexity of coronary artery disease using the SYNTAX score to predict clinical outcome in patients with three-vessel lumen obstruction undergoing percutaneous coronary intervention. Am J Cardiol. 2007; 99:1072–1081.
24. Fukui T, Uchimuro T, Takanashi S. EuroSCORE II with SYNTAX score to assess risks of coronary artery bypass grafting outcomes. Eur J Cardiothorac Surg. 2015; 47:66–71.
25. Møller CH, Penninga L, Wetterslev J, Steinbrüchel DA, Gluud C. Off-pump versus on-pump coronary artery bypass grafting for ischaemic heart disease. Cochrane Database Syst Rev. 2012; (3):CD007224.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download