Abstract
Long QT syndrome (LQTS) is a rare cardiac channelopathy associated with syncope and sudden death due to torsades de pointes and ventricular fibrillation. Syncope and sudden death are frequently associated with physical and emotional stress. Management of patients with LQTS consists of life-style modification, β-blockers, left cardiac sympathetic denervation (LCSD), and implantable cardioverter-defibrillator (ICD) implantation. Prohibition of competitive exercise and avoidance of QT-prolonging drugs are important issues in life-style modification. Although β-blockers are the primary treatment modality for patients with LQTS, these drugs are not completely effective in some patients. Lifelong ICD implantation in young and active patients is associated with significant complications. LCSD is a relatively simple and highly effective surgical procedure. However, LCSD is rarely used.
Long QT syndrome (LQTS) is one of the most important causes of syncope and sudden death in the young.1)2) Effective therapies to prevent syncope and sudden death in such patients are readily available nowadays.3) As syncope and sudden death are due to torsades de pointes and ventricular fibrillation, management of LQTS patients means prevention of development of tachyarrhythmia (Fig. 1). Management modalities consist of life-style modification, β-blockers, left cardiac sympathetic denervation (LCSD), and implantable cardioverter-defibrillator (ICD) implantation. Genotype-phenotype correlations are also important in the management of these patients.
Life-style modification such as avoidance of strenuous exercise, especially swimming or water sports in LQT1 patients, reduction in exposure to abrupt loud noises (alarm clock, phone ringing, etc) in LQT2 patients, and avoidance of drugs that prolong the QT interval in all LQTS patients, should be routinely performed.3)4)5)
According to the 36th Bethesda Conference guidelines, symptomatic patients with LQTS should be limited to Class 1A sports such as brisk walking, hiking, golf, bowling, and skating.6) While asymptomatic genotype-positive/phenotype-negative patients can be allowed to play competitive sports except for swimming in LQT1 as the risk of sudden death is not zero.6) However, according to the more restrictive European Society of Cardiology guidelines, all LQTS patients should be disqualified from all competitive sports based on the QTc cutoff (>440 ms in males, >460 ms in females), whether symptomatic or asymptomatic.7)
Currently, there is a debate regarding participation in competitive sports among experts, especially in genotype-positive/phenotype-negative patients. Some experts think that these guideline-based recommendations for disqualification are excessive and patients may participate in competitive sports safely.8) Recently revised recommendations allow some consideration of competitive sports after ensuring that effective treatment and appropriate precautionary measures such as automatic external defibrillators and personnel trained in basic life support are available, especially among asymptomatic genotype-positive/phenotype-negative patients.8)9) Many patients with ICDs including those with LQTS can engage in vigorous competitive sports without significant morbidity and mortality.10) Recommendation for genotype-positive/phenotype-negative patients is also influenced by physicians' personal exercise habits; less-active physicians are more likely to restrict exercise.11) Risk of cardiac events during sexual activity seems to be extremely low.12)
The development of cardiac events in LQT2 patients is associated with emotional stress and sudden exposure to auditory stimuli, such as noise from telephones, alarm clocks, and crying babies; hence, these sounds should be avoided.4)13) As the QTc interval in LQT2 patients is also dependent on the serum potassium level, the potassium level should be maintained within normal limits. Special care is needed in postpartum women as the risk of cardiac events is increased during the 9-month postpartum period, especially in LQT2.4)14) The 9-month postpartum period is associated with a 2.7-fold increased risk of cardiac events and a 4.1-fold increased risk of life-threatening events when compared with the preconception period. As sleep disturbance and emotional stress in mothers of nursing infants may trigger cardiac events, it is recommended that other family members should take care of infants at nighttime without disturbing the mothers.15)
Some patients with LQTS also suffer from asthma. Asthma comorbidity in LQTS patients is associated with increased risk of cardiac events, which is diminished after initiation of β-blocker therapy.16) β2-agonist therapy in asthmatic LQTS patients increases the risk of cardiac events.17) Asthma in LQTS patients may mean a more severe gene mutation since KvLQT1 seems to be essential for chloride secretion in tracheal epithelial cells.18) Intravenous aminophylline and salbutamol should be avoided and inhaled anticholinergic medication, corticosteroids, intravenous magnesium, and leukotriene receptor antagonists may be considered as alternatives.19)
All patients should avoid exposure to QT prolonging drugs that block the Ikr current. The list of such drugs is available at www.crediblemeds.org and it should be given to the patient and/or family members. Herbal medicines including liquorice20) and grapefruit21) should be avoided. The effect of oral contraceptives seems to be neutral.22) LQTS patients treated with attention deficit/hyperactivity disorder medications showed increased risk of cardiac events, particularly syncope, and this risk is increased in male patients.23)
β-blockers are the primary therapy since the mid-1970s.1)2)24)25) A recent study showed that β-blockers were still the first-line therapy in 76% of European centers.26) β-blockers were associated with a significant reduction in cardiac events in LQTS probands and in the affected family members; 0.97±1.42 to 0.31±0.86 and 0.26±0.84 to 0.15±0.69 events per year, respectively.24) Unfortunately, cardiac events continue to occur while patients are taking the prescribed β-blockers, especially in symptomatic patients; 32% of symptomatic patients will have a cardiac event over 5 years, and 14% of patients with a prior cardiac arrest will have a recurrence within 5 years.24)
As β-blockers are extremely effective in LQT1, β-blocker noncompliance and use of QT prolonging drugs are responsible for almost all life-threatening "β-blocker failures" in LQT1.4)27) Thus, the differentiation between "β-blocker failure" and "patient failure" is important in the management of symptomatic patients after β-blocker treatment. β-blockers are more effective in LQT1 than in LQT2 or LQT3.2)4)28)
β-blockers are very effective in preventing exercise-triggered cardiac events, but they have no significant effect on arousal- or sleep/rest-triggered cardiac events in LQT1 or LQT2 patients.29)30) However, β-blockers should be administered to patients with non-exercise related cardiac events since subsequent exercise-triggered events may still occur in this population.29)
β-blockers ingested by a mother are transmitted to the nursing infant through milk. As β-blocker is effective in preventing cardiac events in the postpartum period, β-blocker medication should be continued during this period as the benefit to the mother far outweighs the negligible risk to the nursing infant.14)
β-blocker-induced symptomatic bradycardia is an extremely rare event if the dosage is gradually increased. Side effects of β-blockers are loss of prowess, fatigue, weight gain, depression, and aggravation of asthma.27) However, bronchial asthma is no longer considered an absolute contraindication to β-blockers.31) β-blockers are also associated with the development of clinically important hypoglycemia in young children, especially those with LQT2.32)33)
Long acting β-blockers in maximally tolerated doses are recommended and abrupt discontinuation should be avoided. Initially, all β-blockers were considered to be equally effective.24) Recently, some experts suggested that not all β-blockers are equally effective in preventing cardiac events, although clear evidences supporting this issue seem to be limited. They believe that propranolol (2-5 mg/kg/day) and nadolol (1-2 mg/day/kg) are definitely more effective than metoprolol, and probably atenolol.34)35)36)37) However, another large study (n=1530) showed that atenolol, metoprolol, propranolol, and nadolol were equally effective in reducing the risk of a first cardiac event in LQTS.38) Their efficacy differed with the genotype; nadolol was the only β-blocker associated with a significant risk reduction in LQT2 and propranolol was the least effective drug in the high-risk group of patients who experienced cardiac events during β-blocker therapy.38) The cause of this discrepancy is not clear. Probably the differences in clinical characteristics of the patients and both dosage and dosing intervals of each β-blocker may have caused this discrepancy. Nadolol is not available in many countries including Korea.
LCSD is a rarely performed procedure but it is quite effective.26) It has been described as having "near ICD levels of protection with less comorbidity".39) LCSD's anti-fibrillatory effect is greatest in LQT1, followed by LQT2 and LQT3.40)41) Dr. Ackerman37) mentioned that none of his patients with single LQT1 mutations experienced a cardiac event after undergoing LCSD and the comorbidities associated with LCSD are much less than those related to ICD implantation. LCSD plays an important role in the proper management of LQTS patients as it could reduce the huge gap between a relatively simple β-blocker medication only and additional ICD implantation. This procedure reduces norepinephrine release at the ventricular level and raises the threshold for ventricular fibrillation without any reduction in cardiac contractility or heart rate.42)43)
Nowadays, LCSD is usually performed in very-high risk infants and small children to serve as a "bridge to an ICD." The usual procedure is high thoracic left sympathectomy and involves ablation of the lower half of the stellate ganglion along with T2 to T4. The results of LCSD are heavily influenced by the experience of the surgeon, as complete resection of the lower half of the left stellate ganglion is critical to the antifibrillatory effects of LCSD.39)41)
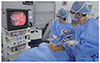
The largest study on LCSD (n=147) showed a 91% reduction in cardiac events per patient during a mean follow-up of 8 years.41) The majority of the patients were at high risk of cardiac events with an extremely prolonged QT interval (mean QTc 543±65 ms). The recently introduced videoscopic LCSD is associated with a short hospital stay and less morbidity (Fig. 2). This procedure is quite similar to sympathectomy for hyperhidrosis, and most Korean chest surgeons are familiar with sympathectomy. The surgical time is usually less than 1 hour, and the patient can be discharged within a few days in experienced centers.
Whenever cardiac events recur in patients on β-blockers, LCSD should be strongly considered. The current indications are as follows: 1) patients with (frequent) appropriate ICD shocks, 2) patients with cardiac events during adequate β-blocker therapy, 3) β-blocker intolerant patients, and 4) very young and/or small patients who are considered technically inappropriate for ICD implantation.26)41) LCSD is especially effective in patients with β-blocker noncompliance and intolerance.40)41) Prophylactic LCSD in selected patients may improve the quality of life resulting from medication-related side effects.40) Unfortunately, approximately 50% of high risk patients have experienced more than one cardiac event after LCSD.40)41) Hence, LCSD must not be viewed as curative or as an ICD-alternative for high risk patients. The most common side effects of LCSD are dry left arm and face and profuse sweating on the right side.44) Complications such as Horner's syndrome or droopy eyelid is very rare and mostly transient.41) A recently published study showed that most of the patients or their parents were satisfied with LCSD and would recommend LCSD to another patient.44)
The usual indications of ICD implantation are patients who have survived a cardiac arrest, patients with syncope despite adequate β-blocker therapy (and LCSD), and some patients considered as high risk with a very long QTc interval (>550 ms), or signs of electrical instability such as T-wave alternans.3)45) The 2013 HRS/EHRA/APHRS guidelines do not recommend ICD implantation in asymptomatic LQTS patients who have not been tried on β-blocker therapy except under "special circumstances"; 1) QTc >550 ms in a patient other than LQT1; 2) LQT2 women with QTc >500 ms; 3) Jervell and Lange-Nielsen syndrome diagnosis; 4) strong family history of LQTS.3) The risk for appropriate shocks in primary prevention patients with LQTS approached zero.46) The current guidelines do not recommend ICD implantation in patients with acquired LQTS. However, it may also be beneficial in acquired LQTS patients as one study showed that 44% of patients with acquired LQTS and ICD received appropriate ICD shocks during a 7-year follow-up.47)
ICD implantation in young and active patients usually leads to inevitable complications such as inappropriate shocks, lead problems, vascular occlusion, infection, psychological adjustment, and social discrimination.48)49) However, the diagnosis of a symptomatic LQTS, especially LQT3, usually leads to an ICD implantation.2)35) A recent European study showed that "Drugs and ICD implantation" was the first-line treatment of LQTS in 19% of participating centers.26) Recommending ICD implantation in a symptomatic LQTS patient is much easier than not recommending implantation of an ICD in this era of "defensive medicine." The ICD implantation rate seems to be as high as 75% in some centers.50) One recent study with 157 patients showed a trend that the majority of patients had Class II and Class III indications for ICD implantation.48) Although the ICD implantation frequency was highest among LQT3 patients, the greatest "save" rate occurred among LQT2 women, who were assessed to be at high risk.50)
The largest study on ICD (n=233, mean age at implantation=30±17 years) showed that female (77%) and LQT3 (22% of known genotype) patients had a disproportionately high probability of being implanted with an ICD.45) During a follow-up of 4.6±3.2 years, at least 1 appropriate shock was received by 28% of the patients, and adverse events occurred in 25% of the patients. Interestingly, >50% of their patients had not suffered a cardiac arrest and many had not even failed β-blocker therapy. Some patients were even asymptomatic. For proper selection of patients for ICD implantation, a new scoring system based on readily available simple clinical variables has been suggested.45) Studies performed at the Mayo clinic clearly showed that the majority of LQTS patients could be managed effectively without ICD implantation, and LQTS-triggered death did not occur in the >500 LQTS patients managed without an ICD.37)50) Recently, some experts cautiously suggested that patients who survived a cardiac arrest without previous β-blocker therapy can be managed without ICD implantation, especially LQT1 patients. They think that β-blockers and LCSD may suffice in such cases. As dual-chamber ICD was not effective in reducing the rate of inappropriate shocks, the decision to implant a dual-chamber ICD without a pacing indication should be avoided to reduce lead related complications.49) To prevent inappropriate shocks, thoughtful programming is necessary, and it usually requires a ventricular fibrillation only zone, with a cutoff rate >220-240 bpm.3)
Potassium supplementation will increase serum potassium levels and may partially correct the repolarization abnormality in patients with LQT2. Two small studies showed that potassium supplementation with spironolactone resulted in significant QTc shortening.51)52) However, the long-term safety and efficacy is not known considering the risk of hyperkalemia and gynecomastia.
Sodium channel blockers such as mexiletine, flecainide, and ranolazine have been used to a limited extent in high risk LQT3 patients refractory to β-blockers or in patients with recurrent events despite ICD and LCSD therapies.2)3) However, their use is limited due to the following reasons: the response is not consistent and mutation specific, ECG response may not correlate with clinical efficacy, and long-term data are not available.
Life-style modification, β-blockers, LCSD, and ICD implantation are important therapeutic modalities in proper management of patients with LQTS. Prudent consideration is needed before making a decision to recommend an ICD implantation in a young and otherwise active patient. LCSD is still severely underutilized.
Figures and Tables
References
1. Moss AJ, Schwartz PJ, Crampton RS, et al. The long QT syndrome. Prospective longitudinal study of 328 families. Circulation. 1991; 84:1136–1144.
2. Lee YS, Kwon BS, Kim GB, et al. Long QT syndrome: a Korean single center study. J Korean Med Sci. 2013; 28:1454–1460.
3. Priori SG, Wilde AA, Horie M, et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Heart Rhythm. 2013; 10:1932–1963.
4. Schwartz PJ, Priori SG, Spazzolini C, et al. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001; 103:89–95.
5. Choi G, Kopplin LJ, Tester DJ, Will ML, Haglund CM, Ackerman MJ. Spectrum and frequency of cardiac channel defects in swimming-triggered arrhythmia syndromes. Circulation. 2004; 110:2119–2124.
6. Zipes DP, Ackerman MJ, Estes NA 3rd, Grant AO, Myerburg RJ, Van Hare G. Task Force 7: arrhythmias. J Am Coll Cardiol. 2005; 45:1354–1363.
7. Pelliccia A, Fagard R, Bjørnstad HH, et al. Recommendations for competitive sports participation in athletes with cardiovascular disease: a consensus document from the Study Group of Sports Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2005; 26:1422–1445.
8. Johnson JN, Ackerman MJ. Return to play? Athletes with congenital long QT syndrome. Br J Sports Med. 2013; 47:28–33.
9. Ackerman MJ, Zipes DP, Kovacs RJ, Maron BJ. Eligibility and Disqualification Recommendations for Competitive Athletes With Cardiovascular Abnormalities: Task Force 10: The Cardiac Channelopathies: A Scientific Statement From the American Heart Association and American College of Cardiology. J Am Coll Cardiol. 2015; 66:2424–2428.
10. Lampert R, Olshansky B, Heidbuchel H, et al. Safety of sports for athletes with implantable cardioverter-defibrillators: results of a prospective, multinational registry. Circulation. 2013; 127:2021–2030.
11. Christian S, Somerville M, Taylor S, Atallah J. Exercise and β-blocker therapy recommendations for inherited arrhythmogenic conditions. Cardiol Young. 2016; 26:1123–1129.
12. Loar RW, Bos JM, Cannon BC, Ackerman MJ. Sudden cardiac arrest during sex in patients with either catecholaminergic polymorphic ventricular tachycardia or long-QT syndrome: a rare but shocking experience. J Cardiovasc Electrophysiol. 2015; 26:300–304.
13. Wilde AA, Jongbloed RJ, Doevendans PA, et al. Auditory stimuli as a trigger for arrhythmic events differentiate HERG-related (LQTS2) patients from KVLQT1-related patients (LQTS1). J Am Coll Cardiol. 1999; 33:327–332.
14. Seth R, Moss AJ, McNitt S, et al. Long QT syndrome and pregnancy. J Am Coll Cardiol. 2007; 49:1092–1098.
15. Schwartz PJ, Ackerman MJ, George AL Jr, Wilde AA. Impact of genetics on the clinical management of channelopathies. J Am Coll Cardiol. 2013; 62:169–180.
16. Rosero SZ, Zareba W, Moss AJ, et al. Asthma and the risk of cardiac events in the Long QT syndrome Investigative Group. Am J Cardiol. 1999; 84:1406–1411.
17. Thottathil P, Acharya J, Moss AJ, et al. Risk of cardiac events in patients with asthma and long-QT syndrome treated with beta2 agonists. Am J Cardiol. 2008; 102:871–874.
18. Mall M, Wissner A, Schreiber R, et al. Role of K(V)LQT1 in cyclic adenosine monophosphate-mediated Cl(-) secretion in human airway epithelia. Am J Respir Cell Mol Biol. 2000; 23:283–289.
19. Collins S, Widger J, Davis A, Massie J. Management of asthma in children with long QT syndrome. Paediatr Respir Rev. 2012; 13:100–105.
20. Crean AM, Abdel-Rahman SE, Greenwood JP. A sweet tooth as the root cause of cardiac arrest. Can J Cardiol. 2009; 25:e357–e358.
21. Lin C, Ke X, Ranade V, Somberg J. The additive effects of the active component of grapefruit juice (naringenin) and antiarrhythmic drugs on HERG inhibition. Cardiology. 2008; 110:145–152.
22. Abu-Zeitone A, Peterson DR, Polonsky B, McNitt S, Moss AJ. Oral contraceptive use and the risk of cardiac events in patients with long QT syndrome. Heart Rhythm. 2014; 11:1170–1175.
23. Zhang C, Kutyifa V, Moss AJ, McNitt S, Zareba W, Kaufman ES. Long-QT syndrome and therapy for attention deficit/hyperactivity disorder. J Cardiovasc Electrophysiol. 2015; 26:1039–1044.
24. Moss AJ, Zareba W, Hall WJ, et al. Effectiveness and limitations of beta-blocker therapy in congenital long-QT syndrome. Circulation. 2000; 101:616–623.
25. Park YM, Kim SJ, Park CH, et al. Repeated aborted sudden cardiac death with long QT syndrome in a patient with anomalous origin of the right coronary artery from the left coronary cusp. Korean Circ J. 2013; 43:830–833.
26. Hocini M, Pison L, Proclemer A, et al. Diagnosis and management of patients with inherited arrhythmia syndromes in Europe: results of the European Heart Rhythm Association Survey. Europace. 2014; 16:600–603.
27. Vincent GM, Schwartz PJ, Denjoy I, et al. High efficacy of beta-blockers in long-QT syndrome type 1: contribution of noncompliance and QT-prolonging drugs to the occurrence of beta-blocker treatment "failures". Circulation. 2009; 119:215–221.
28. Priori SG, Napolitano C, Schwartz PJ, et al. Association of long QT syndrome loci and cardiac events among patients treated with beta-blockers. JAMA. 2004; 292:1341–1344.
29. Goldenberg I, Thottathil P, Lopes CM, et al. Trigger-specific ion-channel mechanisms, risk factors, and response to therapy in type 1 long QT syndrome. Heart Rhythm. 2012; 9:49–56.
30. Kim JA, Lopes CM, Moss AJ, et al. Trigger-specific risk factors and response to therapy in long QT syndrome type 2. Heart Rhythm. 2010; 7:1797–1805.
31. Viskin S, Halkin A. Treating the long-QT syndrome in the era of implantable defibrillators. Circulation. 2009; 119:204–206.
32. Koponen M, Marjamaa A, Hiippala A, et al. Follow-up of 316 molecularly defined pediatric long-QT syndrome patients: clinical course, treatments, and side effects. Circ Arrhythm Electrophysiol. 2015; 8:815–823.
33. Poterucha JT, Bos JM, Cannon BC, Ackerman MJ. Frequency and severity of hypoglycemia in children with beta-blocker-treated long QT syndrome. Heart Rhythm. 2015; 12:1815–1819.
34. Chockalingam P, Crotti L, Girardengo G, et al. Not all beta-blockers are equal in the management of long QT syndrome types 1 and 2: Higher recurrence of events under metoprolol. J Am Coll Cardiol. 2012; 60:2092–2099.
35. Schwartz PJ, Ackerman MJ. The long QT syndrome: a transatlantic clinical approach to diagnosis and therapy. Eur Heart J. 2013; 34:3109–3116.
36. Schwartz PJ. My Approach to the long QT syndrome(LQTS). Trends Cardiovasc Med. 2015; 25:376–377.
37. Ackerman MJ. My Approach to treatment of the congenital long QT syndromes. Trends Cardiovasc Med. 2015; 25:67–69.
38. Abu-Zeitone A, Peterson DR, Polonsky B, McNitt S, Moss AJ. Efficacy of different beta-blockers in the treatment of long QT syndrome. J Am Coll Cardiol. 2014; 64:1352–1358.
39. Schwartz PJ. Cutting nerves and saving lives. Heart Rhythm. 2009; 6:760–763.
40. Bos JM, Bos KM, Johnson JN, Moir C, Ackerman MJ. Left cardiac sympathetic denervation in long QT syndrome: analysis of therapeutic nonresponders. Circ Arrhythm Electrophysiol. 2013; 6:705–711.
41. Schwartz PJ, Priori SG, Cerrone M, et al. Left cardiac sympathetic denervation in the management of high-risk patients affected by the long-QT syndrome. Circulation. 2004; 109:1826–1833.
42. Schwartz PJ, Snebold NG, Brown AM. Effects of unilateral cardiac sympathetic denervation on the ventricular fibrillation threshold. Am J Cardiol. 1976; 37:1034–1040.
43. Schwartz PJ, Stone HL. Effects of unilateral stellectomy upon cardiac performance during exercise in dogs. Circ Res. 1979; 44:637–645.
44. Antiel RM, Bos JM, Joyce DD, et al. Quality of life after videoscopic left cardiac sympathetic denervation in patients with potentially life-threatening cardiac channelopathies/cardiomyopathies. Heart Rhythm. 2016; 13:62–69.
45. Schwartz PJ, Spazzolini C, Priori SG, et al. Who are the long-QT syndrome patients who receive an implantable cardioverter-defibrillator and what happens to them?: data from the European Long-QT Syndrome Implantable Cardioverter-Defibrillator (LQTS ICD) Registry. Circulation. 2010; 122:1272–1282.
46. Olde Nordkamp LR, Wilde AA, Tijssen JG, Knops RE, van Dessel PF, de Groot JR. The ICD for primary prevention in patients with inherited cardiac diseases: indications, use, and outcome: a comparison with secondary prevention. Circ Arrhythm Electrophysiol. 2013; 6:91–100.
47. Mönnig G, Köbe J, Löher A, et al. Role of implantable cardioverter defibrillator therapy in patients with acquired long QT syndrome: a long-term follow-up. Europace. 2012; 14:396–401.
48. Gaba P, Bos JM, Cannon BC, et al. Implantable cardioverter-defibrillator explantation for overdiagnosed or overtreated congenital long QT syndrome. Heart Rhythm. 2016; 13:879–885.
49. Olde Nordkamp LR, Postema PG, Knops RE, et al. Implantable cardioverter-defibrillator harm in young patients with inherited arrhythmia syndromes: a systematic review and meta-analysis of inappropriate shocks and complications. Heart Rhythm. 2016; 13:443–454.
50. Horner JM, Kinoshita M, Webster TL, Haglund CM, Friedman PA, Ackerman MJ. ,Implantable cardioverter defibrillator therapy for congenital long QT syndrome: a single-center experience. Heart Rhythm. 2010; 7:1616–1622.
51. Compton SJ, Lux RL, Ramsey MR, et al. Genetically defined therapy of inherited long-QT syndrome. Correction of abnormal repolarization by potassium. Circulation. 1996; 94:1018–1022.
52. Etheridge SP, Compton SJ, Tristani-Firouzi M, Mason JW. A new oral therapy for long QT syndrome: long-term oral potassium improves repolarization in patients with HERG mutations. J Am Coll Cardiol. 2003; 42:1777–1782.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download