Abstract
Subcutaneous implantation of a cardiac implantable electronic device is the standard method. Occasionally, subpectoral cardiac implantable electronic device (CIED) implantation via axillary incisions is performed in young female patients for cosmetic purposes. Because subpectoral CIED implantation and augmentation mammoplasty involve the same layer, it is feasible to perform both procedures simultaneously. We report a case of combined subpectoral implantation of an implantable cardioverter-defibrillator and augmentation mammoplasty via the axillary approach in a young female patient with dilated cardiomyopathy and small breasts.
The use of implantable cardioverter-defibrillators (ICDs) is important for preventing sudden cardiac arrest in patients at high risk of fatal ventricular arrhythmias.1) In the past decades, cardiologists paid attention primarily to medical problems and prolongation of the life expectancy of patients with heart failure. Recently, the quality of life as well as survival of such patients has been emphasized. Therefore, the demand for subpectoral implantation of cardiac implantable electronic devices (CIEDs) is increasing in young female patients concerned about their body image. We report a case of combined subpectoral implantation of ICD and augmentation mammoplasty via the axillary approach in a young female patient with dilated cardiomyopathy and small breasts.
A 20-year-old female patient presented to the emergency department because of dyspnea and chest discomfort. Chest radiography showed pulmonary edema and bilateral pleural effusion. Transthoracic echocardiogram revealed an enlarged left ventricular dimension and severe global hypokinesia of the left ventricle (ejection fraction, 20%; Fig. 1). Cardiac magnetic resonance images showed severely decreased left ventricular function and ill-defined delayed enhancement in the septum, both compatible with dilated cardiomyopathy. After 9 months of optimal medical treatment including perindopril, furosemide and spironolactone, cardiac function had not improved. A beta-blocker was not taken due to hypotension after administration of carvedilol. The patient still complained of dyspnea on exertion of New York Heart Association functional class II. Non-sustained monomorphic ventricular tachycardia was detected on 24 h electrocardiogram monitoring (Fig. 2). QRS duration during sinus rhythm was 98 ms on electrocardiogram. ICD implantation was indicated for primary prevention of sudden cardiac arrest. We proposed two options for ICD implantation: subcutaneous or subpectoral implantation via the axillary incision. The patient preferred the latter option, and also requested augmentation mammoplasty for her small breasts. After consulting the plastic surgeon, we performed a combined subpectoral ICD implantation and augmentation mammoplasty procedure via the axillary incision.
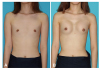
Before augmentation mammoplasty, the volumes of right and left breasts were 46 and 56 mL, respectively, as measured anthropometrically. Under general anesthesia, skin incisions were performed on both axillary creases, and the plane between the pectoralis major muscle and the rib cage were dissected under endoscopic guidance. Two 185 g form-stable gel breast implants (Natrelle; Allergan, Irvine, CA, USA) were implanted into the subpectoral plane of both breasts. The position and shape of the breast implants were inspected in the sitting position. Because the patient's blood pressure decreased in the sitting position, norepinephrine was infused intravenously to maintain mean arterial blood pressure above 80 mmHg. After bilateral breast augmentation by the plastic surgery team, the left axillary vein was punctured via the Seldinger technique. A defibrillating ventricular lead (Durata 7120Q-58cm; Screw type, St. Jude Medical, Valley View Court Sylmar, CA, USA) was inserted into the right ventricle via the 9 Fr guiding sheath and was stably anchored at the right ventricular apex. An atrial lead (Tendril STS 2088TC-52 cm; Screw type, St Jude Medical) was inserted and fixed into the right atrial appendage. The ventricular and atrial leads were connected to the ICD generator (Ellipse DR, St Jude Medical). The ICD generator was implanted into the subpectoral plane immediately above the left breast implant. Combined subpectoral ICD implantation via the left axillary approach (Fig. 3) and augmentation mammoplasty were successfully performed without complications (Fig. 4). After augmentation mammoplasty, volumes of the right and left breasts were 163 and 169 mL, respectively (Fig. 5). The patient was satisfied with her body image after the operation. She did not develop ventricular arrhythmias during her 9-month follow-up.
We present herein a successful case of combined subpectoral ICD implantation and augmentation mammoplasty via axillary incisions in a young female patient who was dissatisfied with her body image.
CIEDs have shown to improve survival and symptoms of patients with heart disease, and their use has been increasing.1)2)3) The use of ICD implantation in young patients due to congenital heart disease, cardiomyopathy, and genetic disorders, such as a long QT syndrome, has also increased.4) Currently, quality of life as well as survival is important. As the number of young patients who require CIED has increased, physicians should be concerned regarding not only patients' medical problems but also esthetic aspects and psychological fitness. In particular, young or female patients are more concerned about their body image and psychosocial distress associated with shock or sudden death rather than older or male patients.5)6) Despite the reduction in the size of CIEDs in the last few decades, routine subcutaneous device implantation in the pectoral area still results in a visible scar and protrusion. Furthermore, protrusion at the anterior chest causes awareness of the device and discomfort with daily activities when using purse straps, bra straps, or seat belts. Therefore, the demand for CIED implantation other than subcutaneously has increased. Recently, in the United States, the application of subpectoral CIED implantation has increased in young female patients having cosmetic concerns.7)8)
Although there are several methods for cosmetic CIED implantation, no nomenclature for these methods has been suggested. We suggest a nomenclature for cosmetic CIED implantation based on a combination of three components: incision for lead insertion, incision for generator insertion, and layer of generator implantation. In previous studies9)10) and real-world practice, four types of cosmetic CIED implantation have been reported: axillary-axillary-subpectoral, axillary-inframammary-submammary, infraclavicular-axillary-subpectoral, and infraclavicular-inframammary-submammary implantation (Fig. 6). The submammary layer lies beneath the mammary glandular tissue and above the pectoralis major muscle. In the present case, we used axillary-axillary-subpectoral implantation. Axillaryaxillary-subpectoral implantation is superior at it results in minimal and invisible scarring.
Complications of subpectoral or submammary CIED implantation are not higher than that of those implanted subcutaneously. Obeyesekere et al.10) reported 20 cases of submammary ICD implantation, and did not note complications related to the implantation site, such as infection and device migration. During a follow-up of 5 years, the incidence of appropriate and inappropriate shock due to the ICD was similar to that reported in other studies. Another study11) compared subcutaneous and submuscular approaches in patients with pectoral ICD. The overall risk of any pocket-related complications was not different between the two groups, and lead complications occurred more frequently in the subcutaneous group.11) Patients' satisfaction and acceptance rates were higher in the submammary or subpectoral group than subcutaneous group.7)9)
A generator is implanted between the pectoralis major muscle and the rib cage via an axillary incision. Although the device is implanted subpectorally or submammarily, esthetic concerns remain in female patients with low body weight and small breasts. A combined subpectoral ICD implantation and breast augmentation surgery has been reported.12) Breast augmentation helps conceal the remaining protuberant chest due to the device. A combined subpectoral CIED implantation and augmentation mammoplasty procedure is feasible, because the layer of CIED implantation is identical to that of breast implantation. Moreover, both can be implanted via the same axillary incision. This combined procedure is usually performed by cooperation between a cardiac electrophysiologist and a plastic surgeon under general anesthesia. In addition, pain control including patient-controlled analgesia is usually necessary after surgery. Therefore, multidisciplinary care by a cardiac electrophysiologist, a plastic surgeon, and an anesthesiologist is important for combined surgery. In particular, vital signs and heart function monitoring during the operation are essential in patients with heart failure. Generator change, ICD removal or repositioning from the subpectoral to subcutaneous area also requires general anesthesia. As mentioned above, the complication rates of CIED implantation in the subpectoral area and other areas are similar. Indeed, in some case series, subpectoral CIED implantations not combined with breast augmentation via an axillary approach were performed successfully.13) However, later procedures should be carefully managed, because the ICD generator and breast implant were implanted in the same subpectoral layer.
Cases of combined subpectoral ICD implantation and augmentation mammoplasty in Asians have not been reported previously. Combined subpectoral ICD implantation and augmentation mammoplasty via the axillary incision is feasible in young female patients with ICD indications and small breasts.
Figures and Tables
Fig. 1
M-mode of the left ventricle. (A) Left ventricular end-diastolic diameter: 66 mm, (B) left ventricular end-systolic diameter: 57 mm. Ejection fraction by M-mode: 20%.
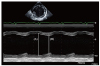
Fig. 2
Non-sustained monomorphic ventricular tachycardia detected by 24 h electrocardiogram monitoring.
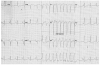
Fig. 3
Placement and fixation of the implantable cardioverter-defibrillator leads through the axillary incision in the surgical field.
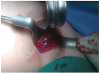
Fig. 4
Chest radiography, posteroanterior (A) and left lateral (B) views after combined subpectoral implantation of an implantable cardioverter-defibrillator and augmentation mammoplasty.
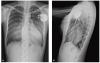
Fig. 5
Clinical photographs before (A) and after (B) the combined subpectoral implantable cardioverter-defibrillator implantation and augmentation mammoplasty procedure. Right breast volume increased from 45 to 163 mL and left breast volume from 56 to 169 mL. There is neither a visible scar nor protrusion on the chest.
References
1. Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002; 346:877–883.
2. Goldberger Z, Lampert R. Implantable cardioverter-defibrillators: expanding indications and technologies. JAMA. 2006; 295:809–818.
3. Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009--a World Society of Arrhythmia's project. Pacing Clin Electrophysiol. 2011; 34:1013–1027.
4. Burns KM, Evans F, Kaltman JR. Pediatric ICD utilization in the United States from 1997 to 2006. Heart Rhythm. 2011; 8:23–28.
5. Starrenburg A, Pedersen S, van den Broek K, Kraaier K, Scholten M, Van der Palen J. Gender differences in psychological distress and quality of life in patients with an ICD 1-year postimplant. Pacing Clin Electrophysiol. 2014; 37:843–852.
6. Vazquez LD, Kuhl EA, Shea JB, et al. Age-specific differences in women with implantable cardioverter defibrillators: an international multi center study. Pacing Clin Electrophysiol. 2008; 31:1528–1534.
7. Kistler PM, Fynn SP, Mond HG, Eizenberg N. The subpectoral pacemaker implant: it isn't what it seems! Pacing Clin Electrophysiol. 2004; 27:361–364.
8. Reiffel JA, Dizon J. Cardiology patient page. The implantable cardioverter-defibrillator: patient perspective. Circulation. 2002; 105:1022–1024.
9. Giudici MC, Carlson JI, Krupa RK, Meierbachtol CJ, Vanwhy KJ. Submammary pacemakers and ICDs in women: long-term follow-up and patient satisfaction. Pacing Clin Electrophysiol. 2010; 33:1373–1375.
10. Obeyesekere MN, Kamberi S, Youngs N, Alison J. Long-term performance of submammary defibrillator system. Europace. 2010; 12:1239–1244.
11. Gold MR, Peters RW, Johnson JW, Shorofsky SR. Complications associated with pectoral cardioverter-defibrillator implantation: comparison of subcutaneous and submuscular approaches. Worldwide Jewel Investigators. J Am Coll Cardiol. 1996; 28:1278–1282.
12. Thomas DE, Murison MS, Anderson MH. Combined subpectoral implantation of a cardioverter defibrillator and breast augmentation surgery in a patient with Emery-Dreifuss muscular dystrophy. Future Cardiol. 2015; 11:293–295.
13. Al-Bataineh M, Sajadi S, Fontaine JM, Kutalek S. Axillary subpectoral approach for pacemaker or defibrillator implantation in patients with ipsilateral prepectoral infection and limited venous access. J Interv Card Electrophysiol. 2010; 27:137–142.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download