Abstract
The concept of J wave syndromes was first proposed in 2004 by Yan et al for a spectrum of electrocardiographic (ECG) manifestations of prominent J waves that are associated with a potential to predispose affected individuals to ventricular fibrillation (VF). Although the concept of J wave syndromes is widely used and accepted, there has been tremendous debate over the definition of J wave, its ionic and cellular basis and arrhythmogenic mechanism. In this review article, we attempted to discuss the history from which the concept of J wave syndromes (JWS) is evolved and current controversies in JWS.
The concept of J wave syndromes was first proposed in 2004 by Yan et al.1) for a spectrum of electrocardiographic (ECG) manifestations of prominent J waves that are associated with a potential to predispose affected individuals to ventricular fibrillation (VF). Although the concept of J wave syndromes is widely used and accepted, there has been tremendous debate over the definition of J wave, its ionic and cellular basis and arrhythmogenic mechanism. In this review article, we attempted to discuss the history from which the concept of J wave syndromes (JWS) is evolved and current controversies in JWS.
The J wave is a positive deflection seen at the end of the QRS complex; it may stand as a distinct "delta" wave following the QRS, or be partially buried inside the QRS as QRS notching or slurring. The earliest description of the ECG changes representing J wave (QRS slurring or notching) in healthy young individuals was by Shipley and Hallaran in 1936.2) In 1953, Osborn published a landmark paper for hypothermia-induced "current of injury" that manifested more prominent J wave in dogs.3) Therefore, J wave is also termed as Osborn wave.
Ionic and cellular basis of J wave remained unclear until 1996 when Yan and Antzelevitch developed a novel experimental preparation, i.e. an arterially-perfused canine ventricular wedge, in which transmembrane action potentials from epicardium, endocardium and midmyocardium (M cells) could be recorded simultaneously with a transmural ECG.4) They demonstrated that a prominent action potential notch in the epicardium mediated by transient outward current (Ito) is responsible for the appearance of J wave on the ECG under a normal activation sequence from the endocardium to the epicardium (Fig. 1). Therefore, J wave is an ECG manifestation an Ito-mediated repolarization component, and its property is determined by the features of Ito which can help to distinguish J wave from depolarization abnormalities resembling J wave (pseudo J wave).5)6) In this landmark study, they pointed out that the so-called "right bundle branch block" in cases of VF by Brugada brothers in 1992 was in fact a prominent J wave.7) Since then, J wave has not been simply viewed as a hallmark of hypothermia, but also linked to VF.
In early 2000s, there were a few of case reports that J wave in inferior leads were associated with idiopathic VF.8)9)10) Intrinic connection via the underlying ionic and cellular basis between the Brugada syndrome (BrS) and idiopathic ventricular fibrillation with a prominent J wave in inferior leads was then discussed.8) The concept of JWS was introduced by Yan et al. in 2004 and 2005.1)11)12) In Yan's initial proposal, JWS are a spectrum of ECG manifestations of prominent J waves that are associated with a risk for the development of VF or sudden cardiac death, including BrS, idiopathic VF with prominent J waves in inferior leads and early repolarization (ER) syndrome by the traditional definition (see below). The ionic and cellular basis linking the ECG manifestations of BrS, the idiopathic VF and ER syndrome is Ito-mediated epicardial action potential notch and phase 2 reentry that severs as the common mechanism underlying the VF development.1)11)12)
In 2008, Haissaguerre et al reported that J point elevation in two consecutive leads in a group of 206 case subjects with idiopathic VF is more common than that in a control group of 412 healthy individuals.13) Since then, JWS has become one of the hottest topics in basic and clinical electrophysiological research.5)6)14)15) However, they redefined J wave or J point elevation to be at least 1 mm (0.1 mV) above the baseline level in two consecutive leads as "early repolarization (ER)" in the study.13) Such a deviation in definition from the traditional ER description has caused confusion in both research and clinical practice involving JWS.
When Shipley and Hallaran described J wave in 1936,2) they also described ST elevation as a normal variant but did not specify if the elevation was related to J wave. In 1961, Wasserburger and Alt16) clearly defined ER as a 'normal precordial RS-T segment elevation variant'. In their definition, ER was characterized as: (1) an elevated take-off of the S-T segment at the J junction of the QRS complex, varying from 1 to 4 mm, relative to the succeeding T-P interval; (2) a downward (should be "upward", the authors might use a wrong word) concavity of the S-T segment; and (3) symmetrically limbed T waves with a large amplitude. This pattern is usually seen in the mid- and left precordial leads V3-V5. The authors also indicated accelerated ventricular repolarization was considered as the mechanism of this ECG pattern. In a review article by Gussak and Antzelevitch in 2000, ER syndrome is defined as a diffuse upward ST-segment concavity ending in a positive T wave in leads V2-V4.17) In other words, J wave and ER were historically considered as two distinguished ECG manifestations.
Haissagueree et al.13) in 2008 defined ER as an elevation of the QRS–ST junction (J point) in at least two consecutive leads, excluding right precordial leads. Since then, a number of publications, including the consensus statement on the diagnosis and management of primary inherited arrhythmia syndromes from HRS/EHRA/APHRS18) and a recent expert consensus paper on ER,19) have adopted this new definition. In contrast to the traditional ER definition, the upwardly concave ST segment elevation is no longer included in the new ER definition (Table 1). This shift in ECG definition of "early repolarization" from traditional one, which primarily focused on ST segment elevation, to QRS terminal J point elevation including Ito-mediated J wave has generated tremendous confusion in almost aspects in basic research as well as in clinical practice.14)20)21)
Firstly, the traditional ER and modern ER (an elevation of the QRS–ST junction) represent respectively two different ECG components. The major differences between the traditional ER and modern ER are shown in Table 1. Therefore, it is not surprising that the prevalence of ER in the general population has increased from 1-2% 16)22) using the classic definition to 6-13% 23)24) following new definition. Furthermore, two large population-based cohort studies23)24) from Europe demonstrated that ER by new definition was associated with an increased risk of cardiovascular mortality in middle-aged subjects. However, a statement or similar ones, like "ER had traditionally been viewed as benign until 2008 when ......" has been commonly seen in many current literatures with use of new ER definition. This apparently defies logic: the authors were trying to compare apples with oranges. Similarly, a recent meta-analysis also indicated that the ER was associated with increased risk and a low to intermediate absolute incidence rate of arrhythmia death.25)
However, the authors didn't analyze the prognostic impact of ER according to different ER definitions. Historically, QRS slurring or J point elevation, i.e. newly defined ER, was never viewed as benign. The classic study by Osborn in 1953 clearly demonstrated that hypothermia-induced J (Osborn) wave was associated with the development of VF in dogs. It has also been known for more than a half century that induced hypothermia for surgical procedures led to VF in humans.26)27) Historically, quinidine was used to prevent VF in patients who required hypothermia for surgical procedures.28) We now know that quinidine prevents and suppress VF in JWS via suppressing Ito.5)14)15)29) Numerous case reports before 2008 had demonstrated a strong link between J wave in ECG leads excluding V1 to V3 and VF 8)9)10)30)31) and such a link via J wave and its underlying mechanism for VF, similar to that in BrS, led to naming JWS in 2004.1)11)12)
Secondly, a shift in the definition of ER from the traditional one which focused on ST segment elevation to an elevation of the QRS–ST junction causes confusion not only in the concept but also in clinical diagnosis of repolarization abnormalities related to idiopathic VF. An elevation of the QRS–ST junction can be a product of either Ito-mediated J wave, a repolarization component,4) or ventricular conduction delay from depolarization abnormalities (Fig. 2). Clearly, ventricular conduction delay (i.e. a pseudo J wave) has nothing to do with "early repolarization". On the other hand, the traditional ER does represent accelerated or "early" repolarization in the ventricular epicardium. While there is no voltage gradient across the ventricular wall during early phase of repolarization, i.e. that the membrane potentials of epicedium and endocardium are at the same level, ST segment remains isoelectric (Fig. 3B, left panel); when the epicardial potential at phase 2 separates early from those of other myocardial layers, a resultant transmural voltage gradient manifests upwardly concave ST segment elevation (Fig. 3B, right panel). Accelerated repolarization in the epicardium is the core meaning of the traditional ER, which can be found in the landmark paper from Wasserburger and Alt16) in 1961: "Accelerated ventricular repolarization has generally be held as the mechanism for this phenomenon (i.e. upwardly concave ST segment elevation)". This can be learnt from Fig. 3 that Ito-mediated action potential notch in the epicardium contributes importantly to accelerated repolarization in the epicardium. For example, it can be certain to say that a QRS slurring or a delta wave-like deflection at QRS-ST junction in the presence of upwardly concave ST segment elevation is an Ito-mediated J wave (Fig. 3A); however, it is inappropriate to state that a QRS slurring or a delta wave-like deflection at QRS-ST junction is always a J wave or an early component of repolarization because a QRS slurring or a delta wave at QRS-ST junction can result from conduction delay.
The major difference between Ito-medicated J wave and pseudo J wave (intra-ventricular conduction delay) is their distinguished responses to changes in heart rate. Delayed conduction becomes aggravated during prematurity of fast heart rate, which leads to an accentuation of the QRS notch; whereas repolarization abnormality weakens, leading to a decreased or disappeared Ito-mediated J wave during tachycardia or premature beats. And traditional ER or Ito-mediated J wave is usually accentuated during slower heart rate or long pauses (Fig. 4). Very recently, Aizawa et al showed that "J wave" in a general population was not related to idiopathic VF and augmented in amplitude at shorter RR intervals,32) which, we believe, is the pseudo J wave. Table 2 lists the factors that distinguish Ito-medicated J wave and pseudo J wave.
Therefore, the cause-effect relationship between Ito-mediated action potential notch in the epicardium and a change in ST segment plays a center role in rate-dependent changes in ST segment, T wave and arrhythmogenesis in JWS.
It would be expected that a repolarization abnormality is the primary mechanism underlying the development of VF in the setting of prominent Ito-mediated J wave. This is simply because Ito-mediated J wave is a component of ventricular repolarization. However, whether a repolarization or depolarization abnormality as the mechanism underlying JWS has been an issue of controversy in the case of BrS.
The repolarization hypothesis maintains that a net outward current shift in the balance of currents in right ventricular epicardium leads to repolarization abnormalities resulting in the development of phase 2 reentry, which generates closely-coupled premature beats capable of precipitating VF.5)11)29) In contrast, the depolarization hypothesis maintains that slow conduction in the right ventricular outflow tract plays a major role in the development of the electrocardiographic and arrhythmic manifestations of the syndrome.
The repolarization hypothesis of Ito-mediated epicardial AP notch and its manifestations on the ECG as the cellular and ECG basis of JWS has been supported by a number of bench as well as clinical studies.6)11)29)33)34) In the canine right ventricular wedge preparation, when Ito-mediated epicardial AP notch is deep enough and close to the threshold potential for L-type calcium current (ICa,L) activation, complete loss of epicardial AP dome may occur. During transition to complete loss of epicardial AP dome, a few of interesting electrical alterations occur: (1) the dome is markedly delayed immediately prior to its complete loss, resulting in paradoxical AP prolongation and so called "down slope ST segment elevation", which in fact is a giant J wave, followed by a negative T wave on the ECG (Fig. 4); (2) once the epicardial AP dome is completely lost, AP duration shortens by about 40%,29)35) causing a marked increase in transmural dispersion of repolarization; (3) complete loss of the dome is often heterogeneous across the epicardium: i.e. complete loss of the dome with marked AP shortening occurs in some areas, but the delayed AP dome remains in others.29)35) Due to a marked difference in AP duration and the property the delayed dome similar to early afterdepolarization, the dome may produce a new AP in the areas where complete loss of epicardial AP is present, leading to formation of short-coupled ectopic beats capable of initiating VF (Fig. 5). Because it is propagation of the dome at AP phase 2, it is termed as phase 2 reentry. Phase 2 reentry is the initiator for VF in all of JWS regardless of J wave locations on the ECG. It should be emphasized that a short-coupled ectopic beat on the T wave per se is a strong piece of evidence supporting a repolarization abnormality. There are none of known depolarization abnormalities, for examples arrhythmogenic right ventricular cardiomyopathy (ARVC) or scar related ventricular arrhythmias, that are featured by an initial trigger beat on T wave. The factors supporting a repolarization abnormality as the arrhythmogenic mechanism in the BrS are summarized in Table 3.
The most compelling evidence in support of a depolarization abnormality as the mechanism for BrS comes from a study by Nademanee et al.36) In this study, they found that there were late potentials and fractionated bipolar electrograms in the epicardial sites of the right ventricular outflow tract (RVOT) of BrS patients and that radiofrequency ablation of these sites significantly reduced the arrhythmia-vulnerability and ECG-manifestation of the disease.36) These authors believed that slow conduction within the RVOT is the mechanism underlying VF in BrS as well as the basis for the ameliorative effect of ablation therapy.36) However, Szel and Antzelevitch recently suggested that the late potentials and fractionated bipolar electrograms within the RVOT may be not necessarily the manifestation of slow conduction and that late potentials and fractionated electrogram activity could be the consequence of concealed phase 2 reentry and regional desynchronization in the appearance of the second action potential upstroke, secondary to accentuation of the epicardial action potential notch.37) Ablation of the epicardial sites of phase 2 reentry in the canine ventricular wedge model of the Brugada syndrome markedly diminishes the manifestation of J waves and ST segment elevation and abolishes all arrhythmic activity.37) This indicates that ablation as a method can eliminate not only a depolarization abnormality but also a repolarization abnormality. Most recently, Nademanee et al. found that post-mortem "BrS" patients are associated with interstitial fibrosis and reduced gap junction in the epicedial surface of RVOT.38) However, BrS was not definitely diagnosed in these so called post-mortem "BrS" patients. In addition, the cause-effect relationship between these abnormalities and arrhythmogenesis was not established.
The key rationale why JWS were initially proposed in 2004 and 2005 1)11)12) is that Ito-mediated J wave links BrS, traditional ER and idiopathic VF with prominent J wave in inferior leads. Although this concept is widely accepted, some authors still believe that the Brugada wave is different from J wave in leads excluding V1 to V3.
Coved ST segment elevation in right precordial leads V1 to V3, which is later termed as type 1 Brugada wave, was initially considered as right bundle branch block (RBBB) by Brugada brother in 1992.7) Three years later, Gussak et al.39) first called the coved ST segment elevation as J wave in a patient with recurrent syncope. In 1996, Yan and Antzelevitch pointed out that the coved ST segment elevation in V1 to V3 is in fact a prominent Ito-mediated J wave.4) In patients with global J waves on the 12 lead ECG, J wave in leads excluding V1 to V3 and the Brugada wave changed parallel in response to changes in heart rate.10) Fig. 6 is a good example to demonstrate the counterparts of type 1 and type 2 Brugada waves in a left precordial lead. We recently did a survey using the upper tracing of the Fig. 6A among 27 electrophysiologists from USA, China, Israel, Belgium, Spain, Poland. Twenty-four of them diagnosed Brugada type 2 and type 1 as indicated in the Figure. This indicates that even those electrophysiologists who do not consider the Brugada wave as the counterpart of J wave in V1 to V3 diagnosed the Brugada wave derived from the waves in a left precordial lead that are called J wave!
Similarities and differences between the Brugada wave and J wave in leads excluding V1 to V3 are listed in Table 4. The major difference between the Brugada wave and J wave in leads excluding V1 to V3 is their response to temperature. Hypothermia induce J wave in leads excluding V1 to V3 more often than the Brugada wave. In contrast, fever can induce the Brugada wave but not J wave in leads excluding V1 to V3. The mechanisms underlying the differences are not fully understood, and further basic experiments and clinical studies are required to unlock the puzzles. There are several confounding factors that may contribute to the differences like lead locations in which some have interactions between left and right ventricles, differences in Ito densities and gene mutations. Based on the above discussion, we believe that: type 2 Brugada wave equates J wave (excluding V1 to V3) plus upwardly concave ST segment elevation; type 1 Brugada wave is an amplified J wave (excluding V1 to V3) that is accompanied by a negative T wave.
In summary, J wave and ER were historically considered as two distinguished ECG manifestations. Recent shift in ECG definition of "ER" from traditional one, which primarily focused on ST segment elevation, to QRS terminal J point elevation or slurring which can result from Ito-mediated J wave or intraventricular conduction delay (i.e. pseudo J wave) has caused confusion in both research and clinical practice involving JWS. Although there may be depolarization abnormalities in JWS particularly in the Brugada syndrome, such depolarization abnormalities are not the mechanism for VF initiated by a short-coupled R-on-T ectopic beat. Phase 2 reentry from a repolarization abnormality is the common mechanism underlying VF in all of JWS.
Figures and Tables
Fig. 1
The sequence of ventricular activation influences the appearance of J wave in a transmural ECG recorded from coronary-perfused canine left ventricular wedge preparation. (A) Stimulation of the endocardial (Endo) surface causes the epicardial (Epi) surface to be activated last. In such case the J wave is aligned with Ito-mediated epicardial AP notch. (B) Stimulation of the epicardial surface activates it before the endocardial surface. This causes the epicardial AP notch to occur simultaneously with the QRS, hiding the J wave. Reproduced from Yan and Antzelevitch4) with permission. (C) Endocardial activation at different locations can cause the J wave to occur at the end of the QRS, manifesting as slurred (left panel) or notched (right panel) QRS. Reproduced from Badri et al.5) with permission.

Fig. 2
(A) Left panel: classical J waves with J-point and ST segment elevation that are likely mediated by Ito, Right panel: possible intraventricular conduction delay masquerading as pseudo J waves presented in the study by Tikkanen et al.40) Reproduced from Tikkanen et al.40) with permission. (B) Two examples of increased pseudo J wave amplitude after shorter RR intervals. (C) An example of right bundle branch block can cause pseudo J waves in inferior leads. IVCD: intraventricular conduction delay, RBBB: right bundle branch block.
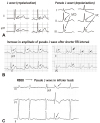
Fig. 3
Cellular basis for the traditional ER. (A) Surface ECG (lead V5) recorded from a 17-year-old healthy African American man. Note the presence of a small J wave and upwardly concave ST segment elevation. (B) Simultaneous recording of transmembrane action potentials from epicardial (Epi) and endocardial (Endo) regions and a transmural ECG in an isolated arterially perfused canine left ventricular wedge. A J wave in the transmural ECG is present due to the action potential notch in the epicardium but not the endocardium. Perfusion of the preparation with pinacidil (2 µmol/L), an ATP-sensitive potassium channel opener, causes partial loss of the action potential dome in the epicardium that separates earlier from the endocardium during phase 2 plateau phase, resulting in upwardly concave ST segment elevation in the ECG resembling the traditional ER. Reproduced from Hlaing et al.12) with permission. ECG: electrocardiography.
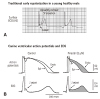
Fig. 4
Frequency-dependent changes in epicardial notch and J wave amplitude. (A) Simultaneously recorded transmural ECG and transmembrane APs from an canine right ventricular wedge: during basic stimulation (S1-S2=4000 ms) there were associated prominent epicardial notch and J wave. Premature stimulation (S1-S2=300 ms) decreased the epicardial notch and J wave amplitude. (B) Plot of epicardial AP notch (□) and J wave (○) amplitude across a range of S1-S2 intervals. Epicardial AP notch and J wave amplitudes changed in parallel to changes in S1-S2 intervals. Reproduced from Yan and Antzelevitch4) with permission. (C) ECG tracings in lead V4-V5 recorded from a 34 year-old Chinese man with J wave syndromes showing prominent J waves that are more accentuated after a long (thick arrows) compared to short (thin arrows) R-R interval. (D) A J-wave-like deflection at the terminal potion of the QRS in a patient with intraventricular conduction delay. In contrary to the J wave in Fig. 4C, prolonged R-R interval allow more time for conduction, attenuating this terminal deflection (thin arrows) compared to the higher amplitude seen in the short R-R interval (thick arrows). Reproduced from Badri et al.5) with permission. ECG: electrocardiography.

Fig. 5
Ito-mediated J wave and ventricular fibrillation (VF) via phase 2 reentry. (A) VF in a male patient with J wave in lead II, note the larger amplitude of J wave in the beat preceding VF, following a longer R-R interval. (B) Phase 2 reentry predisposing to VF in a canine right ventricular wedge preparation in the presence of the K+ channel opener pinacidil. Loss of AP dome in Epi1 but not Epi2 caused propagation of the dome at Epi2 to Epi1, i.e. phase 2 reentry (solid arrows), which manifested a short-coupled R-on-T beats (open arrows) capable of triggering VF. Reproduced from Yan et al.35) with permission. ECG: electrocardiography.
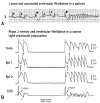
Fig. 6
Example to demonstrate the counterparts of type 1 and 2 Brugada waves. (A) The bottom ECG tracing exhibiting prominent J waves in a left precordial lead V5 was obtained from a 34 year-old Chinese man who survived from idiopathic VF. The J wave wad accentuated after a pause, which was accompanied by a negative T wave. The upper ECG tracing was produced by turning the QRS complex in the bottom ECG tracing (V5) by 180 degree to simulate a pseudo V1 tracing. The upper tracing was then evaluated by 27 electrophysiologists, among whom 24 agreed that there were type 2 and type 1 Brugada waves in this tracing. (B) Type 2 Brugada wave equates J wave plus upwardly concave ST segment elevation in leads excluding V1 to V3; type 1 Brugada wave is the counterpart of a prominent J wave with a negative T wave in leads excluding V1 to V3. ECG: electrocardiography.
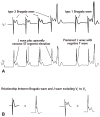
Table 1
The difference between traditional and modern ER

Table 2
The difference between Ito-mediated J wave and Pseudo J wave (i.e. intra-ventricular conduction delay, IVCD)

Table 3
Factors supporting the Brugada syndrome as a repolarization abnormality

Table 4
Comparison of Brugada wave and J wave excluding V1-V3

Acknowledgments
The Sharpe-Strumia Research Foundation and National Natural Science Foundation of China (NSFC-81370289).
References
1. Yan GX, Yao QH, Wang DQ, Cui CC. J wave and J wave syndromes (in Chinese). Chin J Card Arrhythm. 2004; 8:360–365.
2. Shipley RA, Hallaran WR. The four lead electrocardiogram in 200 normal men and women. Am Heart J. 1936; 11:325–345.
3. Osborn JJ. Experimental hypothermia; respiratory and blood pH changes in relation to cardiac function. Am J Physiol. 1953; 175:389–398.
4. Yan GX, Antzelevitch C. Cellular basis for the electrocardiographic J wave. Circulation. 1996; 93:372–379.
5. Badri M, Patel A, Yan GX. Cellular and ionic basis of J-wave syndromes. Trends Cardiovasc Med. 2015; 25:12–21.
6. Antzelevitch C, Yan GX. J-wave syndromes: Brugada and early repolarization syndromes. Heart Rhythm. 2015; 12:1852–1866.
7. Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992; 20:1391–1396.
8. Kalla H, Yan GX, Marinchak R. Ventricular fibrillation in a patient with prominent J (Osborn) waves and ST segment elevation in the inferior electrocardiographic leads: a Brugada syndrome variant? J Cardiovasc Electrophysiol. 2000; 11:95–98.
9. Takagi M, Aihara N, Takaki H, et al. Clinical characteristics of patients with spontaneous or inducible ventricular fibrillation without apparent heart disease presenting with J wave and ST segment elevation in inferior leads. J Cardiovasc Electrophysiol. 2000; 11:844–848.
10. Qi X, Sun F, An X, Yang J. A case of Brugada syndrome with ST segment elevation through entire precordial leads. Chin J Cardiol. 2004; 32:272–273.
11. Shu J, Zhu T, Yang L, Cui C, Yan GX. ST-segment elevation in the early repolarization syndrome, idiopathic ventricular fibrillation, and the Brugada syndrome: cellular and clinical linkage. J Electrocardiol. 2005; 38:26–32.
12. Hlaing T, DiMino T, Kowey PR, Yan GX. ECG repolarization waves: their genesis and clinical implications. Ann Noninvasive Electrocardiol. 2005; 10:211–223.
13. Haissaguerre M, Derval N, Sacher F, et al. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008; 358:2016–2023.
14. Antzelevitch C, Yan GX. J-wave syndromes. from cell to bedside. J Electrocardiol. 2011; 44:656–661.
15. Li GL, Yang L, Cui CC, Sun CF, Yan GX. J wave syndromes: a decade of progress. Chin Med J (Engl). 2015; 128:969–975.
16. Wasserburger RH, Alt WJ. The normal RS-T segment elevation variant. Am J Cardiol. 1961; 8:184–192.
17. Gussak I, Antzelevitch C. Early repolarization syndrome: clinical characteristics and possible cellular and ionic mechanisms. J Electrocardiol. 2000; 33:299–309.
18. Priori SG, Wilde AA, Horie M, et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013; 10:1932–1963.
19. Macfarlane PW, Antzelevitch C, Haissaguerre M, et al. The Early Repolarization Pattern: A Consensus Paper. J Am Coll Cardiol. 2015; 66:470–477.
20. Surawicz B, Macfarlane PW. Inappropriate and confusing electrocardiographic terms: J-wave syndromes and early repolarization. J Am Coll Cardiol. 2011; 57:1584–1586.
21. Bastiaenen R, Behr ER. Benign or malignant, early or delayed: the changing face of early repolarization. Europace. 2012; 14:5–7.
22. Klatsky AL, Oehm R, Cooper RA, Udaltsova N, Armstrong MA. The early repolarization normal variant electrocardiogram: correlates and consequences. Am J Med. 2003; 115:171–177.
23. Tikkanen JT, Anttonen O, Junttila MJ, et al. Long-term outcome associated with early repolarization on electrocardiography. N Engl J Med. 2009; 361:2529–2537.
24. Sinner MF, Reinhard W, Müller M, et al. Association of early repolarization pattern on ECG with risk of cardiac and all-cause mortality: a population-based prospective cohort study (MONICA/KORA). PLoS Med. 2010; 7:e1000314.
25. Wu SH, Lin XX, Cheng YJ, Qiang CC, Zhang J. Early repolarization pattern and risk for arrhythmia death: a meta-analysis. J Am Coll Cardiol. 2013; 61:645–650.
26. Husfeldt E, Secher O. Ventricular fibrillation during hypothermia successfully treated by rewarming and electroshock. Thorax. 1956; 11:67–70.
27. Caldini P. Ventricular fibrillation in induced hypothermia. Postgrad Med J. 1959; 35:538–542.
28. Johnson P, Lesage A, Floyd WL, Young WG Jr, Sealy WC. Prevention of ventricular fibrillation during profound hypothermia by quinidine. Ann Surg. 1960; 151:490–495.
29. Yan GX, Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST-segment elevation. Circulation. 1999; 100:1660–1666.
30. Otto CM, Tauxe RV, Cobb LA, et al. Ventricular fibrillation causes sudden death in Southeast Asian immigrants. Ann Intern Med. 1984; 101:45–47.
31. Aizawa Y, Tamura M, Chinushi M, et al. Idiopathic ventricular fibrillation and bradycardia-dependent intraventricular block. Am Heart J. 1993; 126:1473–1474.
32. Aizawa Y, Sato M, Kitazawa H, et al. Tachycardia-dependent augmentation of "notched J waves" in a general patient population without ventricular fibrillation or cardiac arrest: not a repolarization but a depolarization abnormality? Heart Rhythm. 2015; 12:376–383.
33. Antzelevitch C, Yan GX. J wave syndromes. Heart Rhythm. 2010; 7:549–558.
34. Belhassen B, Glick A, Viskin S. Excellent long-term reproducibility of the electrophysiologic efficacy of quinidine in patients with idiopathic ventricular fibrillation and Brugada syndrome. Pacing Clin Electrophysiol. 2009; 32:294–301.
35. Yan GX, Lankipalli RS, Burke JF, Musco S, Kowey PR. Ventricular repolarization components on the electrocardiogram: cellular basis and clinical significance. J Am Coll Cardiol. 2003; 42:401–409.
36. Nademanee K, Veerakul G, Chandanamattha P, et al. Prevention of ventricular fibrillation episodes in Brugada syndrome by catheter ablation over the anterior right ventricular outflow tract epicardium. Circulation. 2011; 123:1270–1279.
37. Szél T, Antzelevitch C. Abnormal repolarization as the basis for late potentials and fractionated electrograms recorded from epicardium in experimental models of Brugada syndrome. J Am Coll Cardiol. 2014; 63:2037–2045.
38. Nademanee K, Raju H, de Noronha SV, et al. Fibrosis, connexin-43, and conduction abnormalities in the Brugada syndrome. J Am Coll Cardiol. 2015; 66:1976–1986.
39. Gussak I, Bjerregaard P, Egan TM, Chaitman BR. ECG phenomenon called the J wave. History, pathophysiology, and clinical significance. J Electrocardiol. 1995; 28:49–58.
40. Tikkanen JT, Junttila MJ, Anttonen O, et al. Early repolarization: electrocardiographic phenotypes associated with favorable long-term outcome. Circulation. 2011; 123:2666–2673.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download