Abstract
Left atrial intramural hematoma is a very rare complication of radiofrequency ablation procedures. A patient with tachyarrhythmia underwent radiofrequency catheter ablation. Echocardiography performed the following morning showed a large mass in the left atrium, suggestive of intramural hematoma formation. The patient was in a stable condition; therefore, it was decided that follow-up should be conservative and her anticoagulation therapy was continued. The size of the hematoma decreased significantly over the following 50 days. This case highlights a rare complication of a complex catheter ablation procedure in the left atrium that was managed via a noninvasive approach, with which all interventionists should be familiar.
Left atrial intramural hematoma (LAIH) is an exceedingly rare occurrence.1) It is defined as a false, blood-filled cavity or lumen in the left atrial (LA) walls or interatrial septum, creating a new chamber which may or may not be connected with the true LA chamber.2) The few reports available in the existing literature relate LAIH primarily to cardiac procedures such as transcatheter arrhythmia ablation and cardiac surgeries. It is also known to occur spontaneously or in association with amyloidosis, mitral calcification, myocardial infarction, and chest trauma.1)3) The true incidence, etiology, pathophysiology, clinical course, and management of LAIH have yet to be fully elucidated.
We report a case of LAIH after radiofrequency ablation. The purpose of our review is to better define the etiology, clinical course, and management of LAIH in the hope of improving the recognition and outcome of this rare entity.
A 51-year-old woman had recurrent attacks of atrial tachycardia and atrial fibrillation that did not resolve upon treatment with multiple anti-arrhythmic drugs. After an electrophysiological consultation, a transvenous radiofrequency ablation was scheduled. Her medical history was unremarkable. All laboratory values and a baseline echocardiography were normal. The anti-arrhythmic drugs were discontinued 3 days before the procedure.
A single trans-septal puncture was performed under fluoroscopic guidance without any difficulties, and an SL1 sheath (St. Jude Medical Inc., Saint Paul, MN, USA) was introduced into the LA, through which a Lasso catheter was snaked. A7French sheath, 4-pole, 3.5 mm irrigation tip ablation catheter (Biosense Webster Inc., Los Angeles, CA, USA) was also introduced through the same trans-septal puncture and along the Lasso catheter into the LA. A NavX (Navigation & Visualization Technology) system was used for electroanatomic three-dimensional mapping. Ablation was carried out with a Stockert radiofrequency RF generator with temperature limited to 40℃ and power limited to 35 W in the anterior and 20 W in the posterior LA regions.
Incessant atrial tachycardia at a cycle length of 290 ms was present at the outset. Large areas of scar tissue were present in most parts of the LA, especially the anterior and roof areas, but normal signals were recorded in the right atrium, suggesting a diagnosis of isolated LA cardiomyopathy. Tiny pulmonary vein (PV) potentials were evident inside the left superior and inferior PVs. They were easily isolated by an antral line around the PV pairs. Multiple changing circuits of reentrant atrial tachycardia were identified by entrainment mapping and were ablated in the anterior LA and roof. There were no audible pops or increases in impedance. A stable sinus rhythm was restored, and no tachycardia was inducible despite atrial bursts down to 200 ms.
Regarding the anticoagulation regimen, the patient stopped taking Dabigatran 36 hours before the procedure. A bolus of 8000 IU (100 mg/kg) Heparin was given just after the trans-septal puncture, and activated clotting time was maintained at 350-400 seconds throughout the procedure. Enoxaparin (60 mg) was administered 6 hours after the removal of the sheaths. Dabigatran was restarted (110 mg twice daily) the next morning.
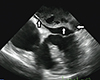
The morning after the procedure, the patient was in sinus rhythm and was feeling well. A routine transthoracic echocardiogram (TTE) revealed a large mass (6×7 cm) occupying most of the LA cavity. Additionally, TTE showed a mitral mean gradient of 6 mmHg, mild tricuspid regurgitation with a mild increase in pulmonary pressure, normal left ventricular dimensions and ejection fraction, and no pericardial effusion. As no further morphological definition of the mass was possible at this stage, transesophageal echocardiography was performed, which revealed a large multi-loculated mass occupying most of the LA space and partially obstructing the left ventricular inflow (Fig. 1). There was no significant obstruction of the venous return. The mass originated from the roof of the LA cavity and extended to the atrioventricular groove. The interatrial septum was intact, delimited by a smooth well-defined wall, and contained some echo-free areas with thrombus-like organization. Also, there was no signal over the interatrial septum in a color-Doppler study.
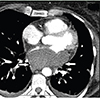
For further understanding of the origin of the mass (intra- vs. extra-cardiac), cardiac computed tomographic angiography (CT-A) imaging was performed, which showed a homogeneous non-enhancing intramural mass consistent with a hematoma located in the posterosuperior LA wall. There was no evidence of stenosis of the PVs or obstruction (Fig. 2).
The patient's hemodynamic status remained stable. After considering the risk of expansion of the hematoma versus the risk of thromboemboli after an extensive LA ablation, we decided to pursue a watchful follow-up while continuing anticoagulation with intravenous unfractionated Heparin. The patient remained in the hospital for the follow-up. Serial TTE showed a stable or improving LA hematoma. A mild reactive pericardial effusion appeared. She was discharged on postoperative day 3 in sinus rhythm on Dabigatran (110 mg twice daily). The size of the hematoma decreased significantly during the following 8 days. A subsequent follow-up TTE in an outpatient clinic 50 days after discharge revealed significant resolution of the hematoma (Fig. 3).
Intramural hematoma is a very uncommon cause of LA masses.4) Complex radiofrequency ablation procedures for the treatment of LA arrhythmias, including atrial fibrillation, have a 6% rate of major complications such as pericardial effusion, embolic events, pulmonary vein dissection, and bleeding secondary to anticoagulation. Intramural atrial hematomas are extremely rare.5)6)
Varying presentation and clinical course are unique characteristics of LAIH. In fact, prompt diagnosis of LAIH tends to be difficult, given its often confusing clinical presentation with hemodynamic instability.2)
Although symptoms usually occur during the 24 hours after a procedure,1) relatively gradual development of the hematoma has also been reported.5) A presumptive diagnosis of LA masses is most often made on the basis of the results of three imaging modalities, i.e. echocardiography, computed tomography, and cardiac magnetic resonance imaging.7) In our patient, although echocardiography was useful for the initial documentation of the hematoma, CT-A was necessary to show not only the size but also the extent of the mass. Knowledge of these features was critical in this case to ensure that the PVs were not compromised. The CT-A scan confirmed that the hematoma was intramural and not intraluminal. Consequently, the patient was carefully observed, and no surgical intervention was undertaken.
Because the clinical course and outcome of LAIH are poorly understood, no definitive criteria exist to guide the management of this rare entity. However, operative intervention is of course indicated if hemodynamic collapse exists.2)
Although in previous reports, prompt surgical repair was undertaken in some patients, it may not be necessary in cases of LAIH with stable hemodynamic status, as was seen in our patient. Therefore, hemodynamic instability, which is indicative of a rapid expansion of the hematoma with the occlusion of the LA cavity and PV or mitral inflow obstruction, may be the critical determinant of the necessity for surgical repair.
The pathogenic mechanism of LAIH is still not clear. Kelly et al.6) posited that transseptal puncture is the main culprit. Nonetheless, as was observed in our patient, LAIH most frequently occurs along the posterior wall of the LA and the atrioventricular groove, both of which provide naturally weakened areas vulnerable to overdistension and forceful manipulation.2) Consequently, we speculate that the hematoma could have been a result of traumatic catheter manipulations in the weakened areas. Specifically, the underlying scarred substrate of the LA may have contributed to the intimal tear in our patient. Thermal injury has been hypothesized as well. In our patient, however, a very limited amount of burns at a low power were applied in the posterior LA.
Another interesting observation is that the resolution of the hematoma was not prevented by continuation of anticoagulation therapy in our patient.
In conclusion, this case highlights the need for careful vigilance in complex radiofrequency ablation procedures. LAIH is an unexpected complication that is rarely reported. This case also highlights the usefulness of cardiac imaging modalities, specifically CT-A, in the noninvasive evaluation of LA masses and the final diagnosis of intramural hematomas. We also demonstrated that conservative management and regular echocardiographic observation should be considered in hemodynamically stable cases, and anticoagulation therapy can be continued.
Figures and Tables
Fig. 1
A 4-chamber view of the transesophagial echocardiograph showing a large multi-loculated mass occupying most of the left atrial space (arrows) and partially obstructing the left ventricular inflow
Acknowledgments
The authors would like to thank Mr. Pedram Amozadeh for his kind assistance in writing the article in English.
References
1. Lanfranchi A, Gelpi G, Rossi RS, Lemma M. A fast-growing obstructive left atrial intramural hematoma causing acute prolonged chest pain. Interact Cardiovasc Thorac Surg. 2009; 9:363–365.
2. Fukuhara S, Dimitrova KR, Geller CM, Hoffman DM, Tranbaugh RF. Left atrial dissection: an almost unknown entity. Interact Cardiovasc Thorac Surg. 2015; 20:96–100.
3. Shaikh N, Rehman NU, Salazar MF, Grodman RS. Spontaneous intramural atrial hematoma presenting as a left atrial mass. J Am Soc Echocardiogr. 1999; 12:1101–1103.
4. Gual-Capllonch F, Arce J, Serés L, et al. Left atrial intramural haematoma associated with mitral annular calcification. Eur J Echocardiogr. 2010; 11:E18.
5. Kurek C, Gwechenberger M, Richter B, Binder T, Loewe C, Gössinger H. Intramural left atrial haematoma mimicking cardiac tamponade after catheter ablation of atrial fibrillation. Europace. 2009; 11:667–668.
6. Kelly S, Bicknell SG, Sharma S. Left atrial wall hematoma after radiofrequency ablation for atrial fibrillation. AJR Am J Roentgenol. 2006; 186:1317–1319.
7. Sah R, Epstein LM, Kwong RY. Images in cardiovascular medicine. Intramural atrial hematoma after catheter ablation for atrial tachyarrhythmias. Circulation. 2007; 115:e446–e447.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download