Abstract
Background and Objectives
Adenosine triphosphate (ATP)-sensitive potassium (KATP) channels play an important role in myocardial protection. We examined the effects of thromboxane A2 on the regulation of KATP channel activity in single ventricular myocytes.
Subjects and Methods
Single ventricular myocytes were isolated from the hearts of adult Institute of Cancer Research (ICR) mice by enzymatic digestion. Single channel activity was recorded by excised inside-out and cell-attached patch clamp configurations at −60 mV holding potential during the perfusion of an ATP-free K-5 solution.
Results
In the excised inside-out patches, the thromboxane A2 analog, U46619, decreased the KATP channel activity in a dose-dependent manner; however, the thromboxane A2 receptor antagonist, SQ29548, did not significantly attenuate the inhibitory effect of U46619. In the cell-attached patches, U46619 inhibited dinitrophenol (DNP)-induced KATP channel activity in a dose-dependent manner, and SQ29548 attenuated the inhibitory effects of U46619 on DNP-induced KATP channel activity.
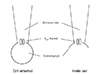
The patch clamp experiment is a technique in electrophysiology that allows the study of single or multiple ion channels in cells; it was developed in the late 1970s and early 1980s by Neher and Sakmann.1) Several configurations of this technique have been introduced, including cell-attached, excised inside-out, and whole-cell patch configuration (Fig.1). In the 'cell-attached' mode, a tight seal is formed between the micropipette and the cell membrane, and the pipette captures the ion channel current flow. Although this configuration does not disturb the intracellular contents, it is difficult to accurately measure the membrane potential and to perfuse into the intracellular space. In the 'excised inside-out' mode, the micropipette is pulled away from the main body of the cell, leaving the formerly intracellular membrane surface exposed to the bath. Even though the cell body is broken in the excised patch, this technique is more likely to regulate the intracellular environment. Cell-attached and excised patch techniques are used to study the behavior of single ion channels in the section of membrane attached to the electrode. However, 'whole-cell' patches allow researchers to study the electrical behavior of the entire cell, instead of single channel currents.2)
Potassium channels (K+ channels) play a crucial role in regulating the action potential of cardiomyocytes. Among K+ channels in the cardiovascular system, the adenosine triphosphate (ATP)-sensitive potassium channels (KATP channels), the first to be discovered in cardiomyocytes,3) have a structure analogous to the inwardly rectifying potassium channel superfamily, and their activity is regulated by the concentration of intracellular ATP metabolites.4)
The activity of KATP channels is regulated by the ratio of ATP/Adenosine Driphosphate or ATP concentration, which is an indicator of intracellular metabolism. Intracellular K+ loss and extracellular K+ accumulation occur within a few minutes of the onset of myocardial ischemia. This is due to the K+ efflux that occurs as KATP channels open when intracellular ATP decreases during myocardial ischemia.5)6)
KATP channel activity simultaneously has a protective effect during ischemia, through vasodilation and the reduction of myocardial contractility, and a negative arrhythmogenic effect caused by the depolarization of the membrane potential.7)8) Due to this, KATP channels are considered to be one of the more interesting ion channels, and research on the substances that regulate the activity of this channel has been increasing.
Thromboxane A2, a member of the eicosanoid family, is a typical vasoconstrictor. Because its effect is generally the opposite of prostacyclin, the balance of these two substances has major implications for the regulation of cardiovascular tension. In particular, a marked increase in thromboxane A2 synthesis during myocardial ischemia-reperfusion has been observed, and it appears to be related to the regulation of cardiac function during myocardial ischemia. If thromboxane A2 is involved in the regulation of KATP channel activity, then, working in opposition to prostacyclin, it decreases the channel activity, increases cardiovascular tension, and likely has an overall negative impact on myocardial ischemia.
We used excised the inside-out and cell-attached patch clamp electrophysiological techniques to investigate the effects of thromboxane A2 on the regulation of KATP channels.
All experiments were performed according to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). The Ethics Committee of Chonnam National University Medical School approved all experimental protocols.
Single ventricular myocytes were obtained from ICR mice (25-35 g). After induction of unconsciousness through cervical dislocation, the thoracic cavity was opened and the heart was extracted.
Using a dissecting microscope at 20× magnification, adipose and connective tissues were removed from the extracted heart in a 4℃, 100% oxygen saturated Tyrode solution (composition: 137 mM NaCl, 5.4 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 0.33 mM NaH2PO4, 10 mM HEPES, 10 mM dextrose, titrated to pH 7.4 with NaOH). After inserting a catheter into the aorta, the aorta was ligated and suspended in a Langendorff device where the coronary arteries were perfused for 5 minutes in a 37℃ Tyrode solution at 1.5 mL/min.
Next, the extracted heart was perfused with a Ca2+-free Tyrode solution until the pulse stopped. With the heart completely relaxed, a Ca2+-free Tyrode solution containing 0.6 mg/mL collagenase (CLS2, Worthington Biochemical Co. Lakewood, NJ, USA) and 0.15 mg/mL protease (type XIV, Sigma-Aldrich Co. St. Louis, MO, USA) was perfused for around 25 minutes; the heart was then perfused for 5 minutes with a high K+, low Cl− solution (composition: 20 mM taurine, 70 mM glutamic acid, 25 mM KCl, 10 mM KH2PO4, 3 mM MgCl2, 0.5 mM EGTA, 10 mM HEPES, 10 mM dextrose, titrated to pH 7.35 with KOH) to remove the remaining enzymes from the heart.
We detached the ventricles from the digested heart, and placed them in a high K+, low Cl− solution. After cutting them into small fragments, they were separated into single ventricular myocytes using light mechanical stimulation with a pipette. After storing the isolated cells in a high K+, low Cl− solution, we examined the inverted microscope (American Optical Co. Buffalo, NY, USA) images, and selected the cells exhibiting no motion and having a distinct rod-shaped outline; these cells were used for all further experiments.
The microelectrodes used in the patch clamp technique were given a resistance of 5–7 MΩ, using 1.5 mm bore diameter borosilicate glass tubing (PG150T-7.5, Clark Electromedical Instruments Co., Edenbridge, Kent, UK) and a micropipette puller (P-97, Flaming/Brown Micropipette Puller, Sutter Instrument Co., Novato, CA, USA). Using a stereozoom microscope (SMZ-2B, Nikon Co., Tokyo, Japan), they were then coated with Sylgard (Dow Corning Co., Midland, MI, USA) up to the area around the microelectrode's terminal, and dried with a laboratory-made coiled heater. Once the terminal endpoints of these microelectrodes had heat reapplied to them and were polished (under an optical microscope [MF-83, Microforge, Narishige Scientific Instrument. Tokyo, Japan]), microelectrodes with a resistance of 3–5 MΩ were used in the experiment.
Single-channel current was recorded using the excised inside-out and cell-attached membrane patch techniques (Fig. 1).9) The electrical signal (at a 2 kHz cut-off frequency) measured with a patch clamp amplifier (Axopatch 200A, Axon Instruments Inc., Union City, CA, USA) was recorded onto a VCR tape with a digital data recorder (VR-10B, Instrutech Co., Longmont, CO, USA).
To analyze single-channel currents, the VCR tape was played, saved to a computer by connecting it to an A/D converter (Digidata 1200 interface, Axon Instruments Inc., Union City, CA, USA), and analyzed using the pClamp program (Version 9, Axon Instruments Inc., Union City, CA, USA). The half-maximum single-unit amplitude threshold method was used to measure the opening and closing times of a single channel.
To measure single-channel currents, an ATP-free K-5 solution was used as an internal, pipette, and bath solution (composition: 140 mM KCl, 2 mM MgCl2, 5 mM EGTA and 10 mM HEPES, titrated to pH 7.2 with HCl). The thromboxane A2 analog, U46619 (9, 11- dideoxy- 9α, 11α- methanoepoxy- prosta- 5Z, 13E- dien- 1- oic acid), and thromboxane A2 receptor antagonist, SQ29548 ([1S-α,α(Z),α,α]]-phenylamino) carbonyl]hydrazino]methyl]-oxabicyclo[2.2.1]hept-yl]-heptenoic acid), used in the experiment were purchased from Cayman Chemical Co. (Ann Arbor, MI, USA). The ATP, Glibenclamide (KATP channel blocker), 2,4-Dinitrophenol (DNP, metabolite restrainer), and other reagents related to the creation of experimental solutions were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Following manufacturers' guidelines, each drug was highly diluted before use in the final experimental solutions.
An excised inside-out patch was prepared under a perfusion of ATP-free K-5 solution. When a holding potential of −60 mV was applied, inverted current channel activity appeared. When 1 mM ATP was added to the intracellular perfusate, the channel activity gradually decreased, and within about a minute no activity could be detected.
When the intracellular perfusate was replaced with an ATP-free K-5 solution, channel activity reappeared. Within about 3 minutes, the channel activity reached similar levels to those seen just after the excised inside-out patch procedure. The addition of glibenclamide (50 µM) caused a gradual weakening of the channel activity, and again, almost no channel activity was seen after about 1 minute. These results suggest that the single-channel activity in the excised inside-out patch was KATP channel activity (Fig. 2).
The current-voltage relationship curve showed inward rectification with a channel conductance of 62±1.3 pS (n=5). The mean single channel current was −3.7±0.26 pA at a −60 mV holding potential (n=5), and there was an inverse relationship between the dwell time of channel opening and the degree of channel activity in a single-channel. The excised inside-out patch experiment targeted the inward current occurring at a −60 mV holding potential (Fig. 3).
Injection of U46619 (0.1–10 µM) into the intracellular perfusate decreased the channel activity in the excised inside-out patch. Inhibition of KATP channel activity by U46619 markedly decreased the frequency of channel openings and mean opening time, but did not affect the size of the single-channel current (Fig. 4).
Furthermore, based on the current-voltage relationship curves of the control and U46619 groups (injection of U46619, 10 µmol/L into the intracellular perfusate), the channel conductance values were 62±1.9 and 63±1.7 pS, respectively (n=5). There were no significant differences between the two groups, and U46619 had no effect on channel conductance (Fig. 5). SQ29548 (0.1 µM) did not attenuate the inhibitory effects of U46619 on KATP channel activity (Fig. 6).
A cell-attached patch in a perfusate of ATP-free K-5 solution was prepared. When a −60 mV holding potential was applied, unlike in the excised inside-out patch, channel activity was weak. When DNP (50 µM) was added, channel activity gradually increased, reaching its peak within 3-5 minutes. When glibenclamide (50 µM) was added, the channel activity gradually weakened. Activity gradually increased when perfused in an ATP-free K-5 solution again. Such channel properties suggest that the single-channel activity within this cell-attached patch experiment was indeed KATP channel activity (Fig. 7).
The mean single channel current was −3.9±0.15 pA at a −60 mV holding potential (n=5), which was similar to the inside-out patch experiment. There was an inverse relationship between the dwell time of channel opening and the degree of channel activity in a single channel. The cell-attached patch experiment targeted the inward current appearing at a −60 mV holding potential.
KATP channel activity was induced by injecting DNP (50 µM) into a bath solution in a cell-attached patch experiment. U46619 was then added (1 and 10 µM) to the perfusate to investigate its effects on channel activity. Injection of DNP caused channel activity to increase, and the activity peaked between 3 and 5 minutes after injection. At the peak of DNP activity, U46619 was added (1 and 10 µM) to the perfusate to investigate its effects on channel activity. This injection resulted in a dose-dependent, gradual decrease in DNP-induced KATP channel activity (Fig. 8). Moreover, the addition of SQ29548 (0.1 µM) attenuated the inhibitory effects of U46619 on DNP-induced KATP channel activity (Fig. 9).
This study was the first to use the patch clamp technique to directly measure the effects of a thromboxane A2 analog on the regulation of KATP channel activity in an isolated single ventricular myocyte.
Known regulators of KATP channel activity include endogenous adenosine, calcitonin gene-regulated peptide, vasoactive intestinal peptide, and endothelium-derived hyperpolarizing factor, as well as the prostaglandin group.10)11)12)13) Recently, nitrous oxide, part of endothelium-derived relaxing factor, was also identified as a regulator of KATP channel activity.13)14)
Several drugs that regulate KATP channel activity have been identified: the first drug was nicorandil, used clinically for the treatment of angina,15) while cromakalim, developed as a coronary relaxant, was the first synthetic potassium channel opener. After that came the development of minoxidil, diazoxide, and others.16) Glibenclamide, a member of the sulfonylurea drug family, is the best known KATP channel blocker, and is also used in the laboratory as a tool for verifying whether KATP channels are responsible for the effects of drugs or endogenous substances.17)
Prostaglandin, an arachidonic acid metabolite that relaxes cardiovascular smooth muscles, increases the KATP channel activity and protects the myocardium. Using the patch clamp technique, Bouchard et al.18) reported that prostaglandin D2, E2, and I2 activate KATP channels in single ventricular myocytes. Jackson et al.19) reported that prostaglandin I2-induced vasodilation in rabbit hearts was partially suppressed by glibenclamide. Additionally, Ju et al.13) reported that prostacyclin increased KATP channel activity in excised inside-out and cell-attached patch experiments in the hearts of white rats. These studies show that prostacyclin plays a role in increasing the KATP channel activity.12)19)
Although thromboxane A2, like prostacyclin, is produced by arachidonic acid via the cyclooxygenase pathway, its role in the cardiovascular system is opposite to that of prostacyclin. Accordingly, if thromboxane A2 is involved in the regulation of KATP channel activity, then having the opposite effects of prostacyclin, it should decrease channel activity, increase cardiovascular tension, and likely have a negative impact on myocardial ischemia. Our results, in particular the finding that a thromboxane A2 analog (U46619) inhibited KATP channel activity, supports this idea.
The results of a previous study showed that thromboxane A2 causes depolarization and suppresses voltage-gated K+ channels in mouse pulmonary arteries,20) and other studies showed that U46619 suppressed the activity of Ca-activated K+ channels in bronchial and coronary smooth muscle,21)22) suggesting the involvement of thromboxane A2 in the regulation of K+ channel activity. However, no study has yet addressed the role of thromboxane A2 in the regulation of KATP channel activity during myocardial ischemia. Xiao et al.23) found that prostacyclin inhibited the ischemia-reperfusion (I/R) injury, and that thromboxane A2 was unrelated to I/R injury in mouse coronary smooth muscles.
Furthermore, there was no inhibitory effect on channel activity from thromboxane A2 receptor antagonist SQ29548 in the excised inside-out patch, but there was significant inhibition in the cell-attached patch. These results imply that U46619 suppresses KATP channel activity, and that this suppression may be influenced by the different pathways within and outside the cell. In other words, our results suggest that U46619 inhibits the activity by working on the target substance around the channel, or the channel itself, in excised inside-out patches, but blocks the activity of the cell membrane receptors in cell-attached patches. However, we don't have any additional results, such as other thromboxane receptor inhibitors or over-expression of SQ29548. We think that our present study suggests the need for further research, along with the different results of channel activity induced by SQ29548 in 2 different patch modes. Future studies will also be required to more clearly establish the role of thromboxane A2 in myocardial ischemia, including the effect of a thromboxane A2 on cell membrane receptors, intracellular signal transduction processes (including second messengers), and the mechanisms that directly regulate channel activity.
In conclusion, our results demonstrate that thromboxane A2 is involved in the regulation of KATP channel activity in single ventricular myocytes isolated from mice via enzymatic separation. Thromboxane A2 reduced the activity of all KATP channels in both excised inside-out and cell-attached patches in a dose-dependent manner.
Figures and Tables
 | Fig. 2Typical ATP-sensitive K+ (KATP) channel activity in an excised inside-out patch (HP=-60 mV). Channel activity appeared immediately after making an excised inside-out patch, and ATP (1 mM) almost completely inhibited the channel activity. Channel activity reappeared when the ATP was washed out with the bath solution, and the KATP channel inhibitor, glibenclamide (50 µM) inhibited the channel activity. ATP: adenosine triphosphate. |
 | Fig. 3Current-voltage relationships of the ATP-sensitive K+ (KATP) channel in the excised inside-out patch at different clamp potentials ranging from −100 to +100 mV (left panel). The current-voltage relationship (right panel) was plotted with the single channel currents in the left panel. HP: holding potential, ATP: adenosine triphosphate. |
 | Fig. 4Effects of thromboxane A2 analog, U46619 (range 1–10 µM) on KATP channel activity in the excised inside-out patch (HP=-60 mV). U46619 inhibited KATP channel activity in a dose dependent manner (upper trace). Both the opening frequencies and the mean open-burst durations were markedly decreased at the times marked (a) and (b) on the expanded scale, but single-channel current amplitudes were not affected (lower two traces). |
 | Fig. 5Influence of thromboxane A2 analog, U46619, on current-voltage, KATP channel relationships in the excised inside-out patch at different clamp potentials. U46619 did not significantly change the channel conductance. pA: pico ampere, CTRL: control. |
 | Fig. 6Influence of thromboxane A2 receptor antagonist, SQ29548 (0.1 µM), on the inhibitory effects of a thromboxane A2 analog, U46619 in an excised inside-out patch (HP=-60 mV). SQ29548 did not influence the inhibitory effects of U46619. |
 | Fig. 7Typical ATP-sensitive K+ (KATP) channel activity in a cell-attached patch (HP=-60 mV). The KATP channel inhibitor, glibenclamide, inhibited the DNP-induced channel activity. ATP: adenosine triphosphate, DNP: dinitrophenol. |
References
1. Neher E, Sakmann B. The patch clamp technique. Sci Am. 1992; 266:44–51.
2. Ackerman MJ, Clapham DE. Ion channels--basic science and clinical disease. N Engl J Med. 1997; 336:1575–1586.
3. Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983; 305:147–148.
4. Trube G, Hescheler J. Inward-rectifying channels in isolated patches of the heart cell membrane: ATP-dependence and comparison with cell-attached patches. Pflugers Arch. 1984; 401:178–184.
5. Kléber AG. Extracellular potassium accumulation in acute myocardial ischemia. J Mol Cell Cardiol. 1984; 16:389–394.
6. Hill JL, Gettes LS. Effect of acute coronary artery occlusion on local myocardial extracellular K+ activity in swine. Circulation. 1980; 61:768–778.
7. Coetzee WA. ATP-sensitive potassium channels and myocardial ischemia: why do they open? Cardiovasc Drugs Ther. 1992; 6:201–208.
8. Antzelevitch C, Di Diego JM. Role of K+ channel activators in cardiac electrophysiology and arrhythmias. Circulation. 1992; 85:1627–1629.
9. Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981; 391:85–100.
10. Kirsch GE, Codina J, Birnbaumer L, Brown AM. Coupling of ATP-sensitive K+ channels to A1 receptors by G proteins in rat ventricular myocytes. Am J Physiol. 1990; 259(3 Pt 2):H820–H826.
11. Quayle JM, Standen NB. KATP channels in vascular smooth muscle. Cardiovasc Res. 1994; 28:797–804.
12. Ju JM, Nah SY, Kim JH. Effect of prostaglandins D2, E2 and I2 on the regulation of KATP channel activity in rat cardiac myocytes. Korean J Physiol Pharmacol. 1999; 3:507–512.
13. Ju JM, Shin DH, Jeong HS, Park HW, Cho JG, Kim JH. Effects of endothelium-derived relaxing factors on the regulation of ATP-sensitive potassium channel activity in cardiac myocytes. Korean Circ J. 2003; 33:420–430.
14. Kubo M, Nakaya Y, Matsuoka S, Saito K, Kuroda Y. Atrial natriuretic factor and isosorbide dinitrate modulate the gating of ATP-sensitive K+ channels in cultured vascular smooth muscle cells. Circ Res. 1994; 74:471–476.
15. Furukawa K, Itoh T, Kajiwara M, et al. Vasodilating actions of 2-nicotinamidoethyl nitrate on porcine and guinea-pig coronary arteries. J Pharmacol Exp Ther. 1981; 218:248–259.
16. Quast U. Potassium channel openers: pharmacological and clinical aspects. Fundam Clin Pharmacol. 1992; 6:279–293.
17. Schmid-Antomarchi H, De Weille J, Fosset M, Lazdunski M. The receptor for antidiabetic sulfonylureas controls the activity of the ATP-modulated K+ channel in insulin-secreting cells. J Biol Chem. 1987; 262:15840–15844.
18. Bouchard JF, Dumont E, Lamontagne D. Evidence that prostaglandins I2, E2, and D2 may activate ATP-sensitive potassium channels in the isolated rat heart. Cardiovasc Res. 1994; 28:901–905.
19. Jackson WF, König A, Dambacher T, Busse R. Prostacyclin-induced vasodilation in rabbit heart is mediated by ATP-sensitive potassium channels. Am J Physiol. 1993; 264(1 Pt 2):H238–H243.
20. Cogolludo A, Moreno L, Bosca L, Tamargo J, Perez-Vizcaino F. Thromboxane A2-induced inhibition of voltage-gated K+ channels and pulmonary vasoconstriction: role of protein kinase Czeta. Circ Res. 2003; 93:656–663.
21. Li QJ, Janssen LJ. Membrane currents in canine bronchial artery and their regulation by excitatory agonists. Am J Physiol Lung Cell Mol Physiol. 2002; 282:L1358–L1365.
22. Scornik FS, Toro L. U46619, a thromboxane A2 agonist, inhibits KCa channel activity from pig coronary artery. Am J Physiol. 1992; 262(3 Pt 1):C708–C713.
23. Xiao CY, Hara A, Yuhki K, et al. Roles of prostaglandin I(2) and thromboxane A(2) in cardiac ischemia-reperfusion injury: a study using mice lacking their respective receptors. Circulation. 2001; 104:2210–2215.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download