Abstract
Background and Objectives
Coarctation of the aorta in adulthood is generally associated with other cardiovascular disorders requiring surgical management. An extra anatomic bypass grafting from the ascending to descending aorta by posterior pericardial approach via median sternotomy could be a reasonable single stage surgical strategy for these patients.
Subjects and Methods
Seven male patients aged between 14-41 years underwent an extra anatomic bypass grafting for coarctation repair concomitantly with the surgical management of the associated cardiovascular disorders via median sternotomy. Preoperative mean systolic arterial blood pressure was 161.8±24.5 mmHg, although the patients were under treatment of different combinations of antihypertensive agents. Additional surgical procedures were: aortic valve replacement (n=4), ventricular septal defect (VSD) closure (n=2), ascending aortic replacement (n=3) and Bentall procedure (n=1). None of our patients have been previously diagnosed or operated on for coarctation. Data were evaluated during their hospital stay and in post-operative follow-up.
Results
The post-operative course was uneventful in all but one patient was re-operated on due to bleeding. There was neither mortality nor significant morbidity during the in-hospital period and all patients were discharged within 5-9 (mean: 6.3±1.5) days. The mean follow up period was 71.83±23 months (range: 23-95 months). Unfortunately one of our patients could not be contacted for a follow up period because of invalid personal data.
An aortic coarctation usually occurs in the isthmic region of the descending aorta where the ligamentum arteriosum originates. It constitutes for 5-8% of the patients having congenital cardiac defects1) and is generally diagnosed and surgically repaired during infancy especially between 2-9 years of age. If not repaired, 25% of the patients are expired by the age of 20; 50% of them by 32 and 90% of them by 58 years of age.2) So, the novel diagnosis of aortic coarctation in adulthood is a rare condition, whereas a concomitant heart disease necessitating repair is seen quite less frequently. Optimal procedure of the surgical repair for these patients still seems to be a subject of debate.
A two stage repair procedure performed via median sternotomy and left thoracotomy3) and a single stage surgical repair procedure of both lesions via sternotomy are the preferences of surgical repair. Extra anatomic bypass grafts from the ascending to descending aorta are used formerly following different routes around the cardiac structure for the repair of coarctation accompanied by a congenital or an acquired heart disease in single stage procedure.4)5)
In this paper, we will share our surgical experience in 7 adult patients with previously uncorrected, postductal aortic coarctation (Fig. 1) accompanied by a congenital or an acquired heart disease necessitating repair, and who have underwent a single stage procedure through a median sternotomy.
We retrospectively analyzed the surgical data and the operative notes of our patients at the Department of Cardiac Surgery of Guven Hospital in Ankara, Turkey and found 7 patients diagnosed with postductal aortic coarctation accompanied by a congenital or an acquired heart disease requiring repair, and underwent a single stage procedure through a median sternotomy. All of the patients underwent an extra-anatomic bypass from the ascending to descending aorta following 3 different routes around the cardiac structure. All of our patients were male and the mean of their ages was 25.86±8.4 years (range: 14-41 years). Hypertension (n=7), family history (n=1) and smoking (n=3) were the pre-operative comorbidity factors among patients. Mean arterial blood pressure was 161.8/94.2±24.5/13.5 mmHg (range: 140-200 mmHg systolic, 70-110 mmHg diastolic) although they were being treated with different combinations of antihypertensive agents. None of our patients had undergone any type of coarctation repair beforehand, but one of them had experienced a cardiac operation for aortic valve replacement (AVR) 18 years ago. Demographic data of our patients are presented in Table 1.
Extra anatomic bypass from the ascending to descending aorta was performed in all of our patients by the indication of an uncorrected coarctation accompanied by a cardiac defect necessitating a repair. Associated cardiac problems concomitant with postductal coarctation repaired via sternotomy were aortic valve regurgitation (n=3), ventricular septal defect (n=2), aortic valve stenosis (n=3), ascending aortic aneurysm (n=4) and prosthetic aortic valve stenosis (n=1) (Table 2).
All of the patients were evaluated by transthoracic echocardiography (TTE) with Doppler, both in pre and post-operative periods, where a transesophageal echocardiographic (TEE) evaluation was performed intraoperatively during all operations. Five patients underwent coronary angiography and ventriculography concomitantly with aortography in pre-operative period and computerized tomography (CT) angiography in the post-operative period eventually. TEE was performed after the routine TTE examination for the patients where CT angiography was not carried out. Mean systolic gradient measured in our patients across the coarctation was 70.5±22.35 mmHg (range: 48-100 mmHg).
Median sternotomy was the only surgical approach method used in all of our patients. Cardiopulmonary bypass (CPB) was instituted by using a two stage or bicaval right atrial and ascending aortic cannulations. Invasive monitorization was performed for following arterial pressures of both upper extremities via brachial or radial arteries and at least one of the femoral arteries for lower extremities. We were ready to perform a femoral arterial cannulation if needed, but there was no need for this intervention due to the performance of collateral arteries (Fig. 2). Ascending aorta was cross clamped to arrest and decompress the heart, as recommended, to accomplish the distal anastomosis of the extra anatomic bypass graft much more comfortably.6) Systemic moderate hypothermia of 28.3±2.33℃ was used meanly in a range of 23.8-30℃ and antegrade and retrograde cardioplegia were both administered to achieve a proper myocardial protection. Then the heart was retracted superiorly and the posterior pericardium was incised longitudinally exposing the descending thoracic aorta parallel to the phrenic nerve meticulously. TEE probe has a critical role here to protect the esophagus from possible injuries that may occur during the posterior pericardial dissections. The dacron tube grafts were anastomosed to the descending thoracic aorta in end-to-side fashion with the use of a side biting clamp. The graft was then clamped, de-aired carefully and positioned towards the ascending aorta for the proximal anastomosis in 3 different routes.
In the first method, after completing the distal anastomosis, the graft was routed around the left side of the heart through the left pleural cavity and lateral pericardial sac anterior to the main pulmonary artery. Four of our patients were operated on with this technique (Fig. 3).
In the second technique, the course of the dacron tube graft was led to the right aspect of the ascending aorta around the right margin of the heart above the inferior vena cava (IVC), which was used in 2 of our patients.
The last technique was performed by routing the graft from the right side of the heart towards the ascending aorta again but this time posterior to the IVC through the oblique sinus; one of our patients was operated in this way.
The sizes of the dacron tube grafts were 16 mm in 6 patients whereas one of them had a size of 18 mm as well.
Cardiac defects requiring repair were achieved after performing the distal anastomosis of the extra anatomic bypass and confirming the accurate hemostasis.
The proximal anastomosis was performed subsequently to the release of the aortic cross clamp by the help of a partial occluding clamp on whether the medial or the right lateral aspect of the ascending aorta depending on the extra anatomic bypass graft's route (Fig. 4). Weaning from CPB in all of our patients was accomplished uneventfully. None of our patients experienced any symptoms of hemodynamic instability and none of them required administration of any type of a positive inotropic drug. Four of our patients had tandem stenosis, suffering from postductal coarctation concomitant with aortic valvular stenosis (AS), but we achieved to protect them from the symptoms of hemodynamic instability by elevating preload and afterload via volume loading carefully, especially during the early periods of weaning from CPB. Measurements of arterial pressures in both upper and lower extremities did not demonstrate any residual gradients post-operatively. Mean cross clamp time was 72.83±20.21 minutes whereas mean CPB period was 123.16±29.74 minutes and the mean duration of the operation was 215.83±55.26 minutes.
There was not any early or late mortality, reported. Only one patient required a revision to control bleeding from the distal anastomosis of the extra anatomic bypass graft, which was the only post-operative morbidity. The mean drainage was 1091.6±318.8 mL. None of the patients experienced any type of neurological deficit. There was no abdominal organ problem or spinal cord ischemia and also left phrenic or left recurrent laryngeal nerve damage and chylothorax was not seen, either. Length of intensive care unit stay was 34.9±11.9 hours. Post-operative hypertension was not seen in any of the patients (mean post-operative systolic blood pressure was 113.3±5.1 mmHg). Decrease in the mean systolic arterial blood pressure was 46.8±22.9 mmHg (range 20.0-80.0 mmHg) post-operatively. In the first month after discharge, hypertension was under control by a triple oral antihypertensive drug complex consisting of a β-blocker, a calcium channel blocker and an angiotensin II receptor antagonist or an angiotensin converting enzyme inhibitor in 3 patients, rest of the patients were treated by a β-blocker only. Combination therapies were cancelled step by step and β-blockers were the only agents to keep hypertension under control in the mid-term and long-term follow-up period. The mean systolic blood pressure during the late follow-up period was 116.16±6.9 mmHg. Mean ventilatory support time was 12.8±5.9 hours. All patients survived and were discharged (period of hospital stay was 6.3±1.5 days) as being well. Five patients had post-operative control CT angiography and TEE was performed for the remaining 2 patients that CT angiography could not be carried out. None of our patients suffered from graft kinking or compression of the tissues. None of them were re-admitted to the hospital because of any late complications. Mean follow up period was 71.83±23 months (range: 23-95 months). Unfortunately contact with one of the patients was lost in the follow up period; we were not able to get in touch with him because of inadequate data about his address and phone numbers (Table 3).
Previously, numerous types of repair techniques were reported in complex forms of coarctation including anatomic repair or extra anatomic bypass grafting.7)8)9)10)11)
Anatomic repair needs a large area to be dissected for a total mobilization of the aorta that may cause bleeding, parenchymal lung injury, chylothorax, laryngeal or phrenic nerve damage due to the neighborhood of this large dissecting area. But, paraplegia is the most dreadful complication in anatomic repair related with long lasting aortic cross clamping time, and older age together with unsuccessful prevention of the patency of extensive collateral circulation. In such cases with complex forms of aortic coarctation, coarctation of the arcus aorta, recurrent adult aortic coarctation and aortic coarctation with an associated cardiac disease requiring surgical repair, extra anatomic bypass by posterior pericardial approach is strongly preferred instead of anatomic repair to protect them from high rates of morbidity and mortality risk.12)13)
In the existence of an acquired or a congenital cardiac defect necessitating repair concomitant with coarctation of aorta, extra anatomic bypass from ascending to descending aorta by posterior pericardial approach provides a surgical repair opportunity by a single stage procedure simultaneously via median sternotomy excluding left thoracotomy and laparotomy incisions.
We performed the technique of Vijayanagar et al.4) in 4 of our patients leading extra anatomic bypass graft around the left side of the heart through the left pleural cavity and lateral pericardial sac anterior to the main pulmonary artery, and then anastomosed to the medial aspect of the ascending aorta proximally. We led the extra anatomic bypass graft around the right lateral aspect of the heart in 3 patients but its course was above the IVC in 2 cases whereas under IVC in 1 of our patients and proximal anastomosis was achieved on the right lateral aspect of the ascending aorta.5)
The route of the extra anatomic bypass graft around the cardiac structure determines the risks of itself. The length of the graft leading around the left margin of the heart is significantly shorter, so possibility of graft kinking and compression on the surrounding tissues especially in the period of adolescence and childhood14) seems to be less than the graft coursing around the right lateral aspect of the heart. Extra anatomic bypass coursing through the right margin of the heart is preferred versus the graft leading around the left margin because it prohibits the risks of resternotomy as the graft is hidden away from the sternum.
We preferred the courses of the grafts depending on this point of view. The mean age of the patients who underwent this single stage procedure was 25.86±8.4 years. All patients had a risk of resternotomy in the rest of their lives. Re-sternotomy would be a serious risk factor for these patients if the graft adhesion occurred at the bottom of the sternum, so we chose the left margin of the heart as the course of a graft only when enough mediastinal space was present between the graft and the sternum. When mediastinal space was not enough to protect the graft coursing through the left margin of the heart, we decided to choose the right aspect of the heart since this course would keep the graft as far as possible from the sternum. The risks of graft kinking and structural compression of the graft should be kept in mind in these cases. The length and the neighborhood of the graft must have been arranged meticulously. As a result of the true choices about the routes of the extra anatomic bypass grafts; we had no graft related problems in our patients.
We preferred to perform a single stage procedure in our patients because both coarctation and the associated cardiac diseases were severely significant, therefore neither the coarctation nor the associated cardiac anomalies could be postponed to be operated via staged procedure. If only coarctation was operated, different symptoms of hemodynamic instability would have occurred due to the effects of cardiac defects whereas if only the cardiac defects were repaired, critical stenosis due to coarctation might have caused a perfusion anomaly distally and left ventricular pressure load resulting in congestive heart failure.
Distal anastomosis of the extra anatomic bypass to the descending thoracic aorta was preferred because exposing the descending aorta just superior to the diaphragm was much easier. Dissecting the descending aorta just above the diaphragm was safer as being very far away from fragile collateral blood vessels. Some authors performed distal anastomosis of the extra anatomic bypass grafts to supraceliac, infrarenal abdominal aorta or even to femoral arteries because they defend that long grafts could be managed and anastomosed much more easily by bending gently. So, it would be possible to avoid the sharp angling and the obstruction of the graft during the distal anastomosis of the descending aorta.10)15)
All of our patients were fully heparinized; therefore we preferred the descending thoracic aorta instead of abdominal aorta for distal anastomosis in order to preclude the extension of the skin incision and hemorrhagic dissections in the abdomen.
Pethig et al.16) performed a single stage procedure via sternotomy for AVR and extra anatomic bypass graft from ascending to descending aorta in 2 patients that had aortic coarctation concomitantly with AS in 1996. Although hemodynamic symptoms were stable initially after weaning from CPB, severe left ventricular failure occurred after fibrillation in a patient. AS and coarctation together can be described by a term of 'tandem stenosis'. In these patients left ventricles were hypertrophied necessitating high perfusion pressures to maintain sufficient coronary flow. Extra anatomic bypass provided a relief on the stenotic isthmic region and this resulted with a decrease in the pressure of the ascending aorta; this pressure was not sufficient enough to supply a proper coronary perfusion for the hypertrophied left ventricle. Global myocardial ischemia occurred due to diminished coronary blood supply but, after administration of positive inotropic agents, especially norepinephrine, adequate pressure for preload and afterload were achieved together with hemodynamic stability.16)
We had 4 patients having aortic coarctation concomitantly with AS undergoing a single stage procedure. We performed weaning from CPB uneventfully without any positive inotropic support by elevating preload and afterload with efficacious volume loading.
There is still no consensus on optimal surgical procedure for adult postductal coarctation patients suffering from associated cardiac diseases requiring repair; but extra anatomic bypass grafting by posterior pericardial approach from ascending to descending aorta via sternotomy seems to be a safe and efficacious surgical method when associated cardiac anomalies require simultaneous operations even in patients older than 70 years of age.17)18)
Figures and Tables
Fig. 1
Pre-operative view of postductal aortic coarctation (white arrow) concomitant with ascending aortic aneurysm (yellow arrow).
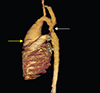
Fig. 2
Collateral arteries originating from the intercostal and internal thoracic arteries in postductal aortic coarctation.
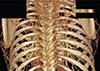
Fig. 3
Proximal anastomosis of the extra anatomic bypass graft (yellow arrow) on the medial aspect of the replaced ascending aorta (white arrow).
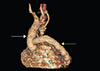
Fig. 4
Extra anatomic bypass graft (yellow arrow) routing from descending (blue arrow) to ascending aorta (white arrow) through the left margin of the heart anterior to the main pulmonary artery.
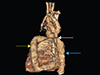
Table 1
Pre-operative demographic data

Table 2
Preoperative diagnosis and concurrent surgical procedures

Table 3
Operative findings

References
1. Kenny D, Hijazi ZM. Coarctation of the aorta: from fetal life to adulthood. Cardiol J. 2011; 18:487–495.
2. Campbell M. Natural history of coarctation of the aorta. Br Heart J. 1970; 32:633–640.
3. Mulay AV, Ashraf S, Watterson KG. Two-stage repair of adult coarctation of the aorta with congenital valvular lesions. Ann Thorac Surg. 1997; 64:1309–1311.
4. Vijayanagar R, Natarajan P, Eckstein PF, Bognolo DA, Toole JC. Aortic valvular insufficiency and postductal aortic coarctation in the adult. Combined surgical management through median sternotomy: a new surgical approach. J Thorac Cardiovasc Surg. 1980; 79:266–268.
5. Powell WR, Adams PR, Cooley DA. Repair of coarctation of aorta with intracardiac repair. Tex Heart Inst J. 1983; 10:409–413.
6. Bigdeli AK, Schmitz C, Kaczmarek I, et al. Combined aortic valve replacement and extra-anatomic aorta ascending-descending bypass for recurrent aortic coarctation. Ann Thorac Surg. 2010; 89:e22–e24.
7. Sweeney MS, Walker WE, Duncan JM, Hallman GL, Livesay JJ, Cooley DA. Reoperation for aortic coarctation: techniques, results, and indications for various approaches. Ann Thorac Surg. 1985; 40:46–49.
8. Edie RN, Janani J, Attai LA, Malm JR, Robinson G. Bypass grafts for recurrent or complex coarctations of the aorta. Ann Thorac Surg. 1975; 20:558–566.
9. Kanter KR, Erez E, Willians WH, Tam VK. Extra-anatomic aortic bypass via sternotomy for complex aortic arch stenosis in children. J Thorac Cardiovasc Surg. 2000; 120:885–890.
10. Robicsek F. "Very long" aortic grafts. Eur J Cardiothorac Surg. 1992; 6:536–541.
11. Izhar U, Schaff HV, Mullany CJ, Daly RC, Orszulak TA. Posterior pericardial approach for ascending aorta-to-descending aorta bypass through a median sternotomy. Ann Thorac Surg. 2000; 70:31–37.
12. Almeida de Oliveira S, Lisboa LA, Dallan LA, Abreu F CA, Rochitte CE, de Souza JM. Extraanatomic aortic bypass for repair of aortic arch coarctation via sternotomy: midterm clinical and magnetic resonance imaging results. Ann Thorac Surg. 2003; 76:1962–1966.
13. Wang R, Sun LZ, Hu XP, et al. Treatment of complex coarctation and coarctation with cardiac lesions using extra-anatomic aortic bypass. J Vasc Surg. 2010; 51:1203–1208.
14. Fedoruk L, Sett SS, Murphy JJ 3rd, LeBlanc JG, Patterson MW. Compression of mediastinal structures treated by extra-anatomic bypass grafting. J Card Surg. 2004; 19:343–345.
15. Sun LZ, Luo XJ, Liu YM. Single-stage treatment of aortic coarctation and aortic valve disease. Asian Cardiovasc Thorac Ann. 2003; 11:208–212.
16. Pethig K, Wahlers T, Tager S, Borst HG. Perioperative complications in combined aortic valve replacement and extraanatomic ascending-descending bypass. Ann Thorac Surg. 1996; 61:1724–1726.
17. Patel Y, Jilani MI, Cho K. Coarctation of the aorta presenting in a 79-year-old male. Thorac Cardiovasc Surg. 1998; 46:158–160.
18. Park JH, Chun KJ, Song SG, et al. Severe aortic coarctation in a 75-year-old woman: total simultaneous repair of aortic coarctation and severe aortic stenosis. Korean Circ J. 2012; 42:62–64.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download