Abstract
Background and Objectives
Subjects and Methods
Results
Figures and Tables
Fig. 1
Flowchart of enrollment and classification of the subjects. KD: Kawasaki disease, IVIG: intravenous immunoglobulin, CAD: coronary artery dilatation.
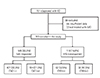
Fig. 2
Mean values of maximum coronary artery Z-scores in IVIG responders and nonresponders. *Analyzed by an unpaired t-test. IVIG: intravenous immunoglobulin.
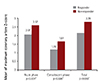
Fig. 3
Frequency of CAD (Z-score≥2.5) in IVIG responders and nonresponders. *Analyzed by a Chi-square test. CAD: coronary artery dilatation, IVIG: intravenous immunoglobulin.
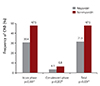
Fig. 4
Severity of CAD in IVIG responders and nonresponders. *Analyzed by a Chi-square test with linear by linear association. No dilatation (Z<2.5) in 463 (65.9%), small dilatation (2.5≤Z<5) in 228 (32.4%), large dilatation (5≤Z<10) in 10 (1.4%), and giant dilatation (Z≥10) in 2 (0.3%). CAD: coronary artery dilatation, IVIG: intravenous immunoglobulin.

Table 1
Clinical characteristics and laboratory findings of IVIG responders and non-responders

Table 2
Multivariate logistic regression analysis for predictors of IVIG non-responders

Table 3

| Responders (n=585) | Non-responders (n=118) | p* | |
|---|---|---|---|
| Kobayashi score5) | 1.73±1.61 | 2.60±2.03 | <0.001 |
| Low risk (0-3) | 509 (87.0)† | 81 (68.6) | |
| High risk (4-6) | 76 (13.0) | 37 (31.4)‡ | |
| Egami score6) | 1.22±1.09 | 1.84±1.38 | <0.001 |
| Low risk (0-2) | 509 (87.0)† | 78 (66.1) | |
| High risk (3-5) | 76 (13.0) | 40 (33.9)‡ | |
| Sano score7) | 0.52±0.70 | 0.91±0.92 | <0.001 |
| Low risk (0-1) | 536 (91.6)† | 85 (72.0) | |
| High risk (2-3) | 49 (8.4) | 33 (28.0)‡ |
Data are presented as either mean±standard deviation or n (%). *Analyzed by an unpaired t-test. †Specificity and ‡sensitivity of each scoring system. The parameters of Kobayashi score5) are as follows: 1) sodium ≤133 mmol/L, 2 points, 2) days of illness at initial treatment≤4 days, 2 points, 3) AST≥100 IU/L, 2 points, 4) % of neutrophil≥80, 2 points, 5) CRP≥10 mg/dL, 1 point, 6) age≤12 months, 1 point, and 7) platelet count≤300×103/mm3, 1 point. The parameters of Egami score6) are as follows: 1) ALT≥80 IU/L, 2 points, 2) illness days≤4 days, 1 point, 3) CRP≥8 mg/dL, 1 point, 4) age≤6 months, 1 point, and 5) platelet count≤300×103/mm3, 1 point. The Sano scoring system7) is as follows: 1) AST≥200 IU/L, 1 point, 2) CRP≥7 mg/dL, 1 point, and 3) total bilirubin≥0.9 mg/dL, 1 point. AST: aspartate aminotransferase, ALT: alanine aminotransferase, CRP: C-reactive protein
Table 4
Clinical characteristics and laboratory findings of CAD (-) and CAD (+) groups

Data are presented as either mean±standard deviation or n (%). *Analyzed by an unpaired t-test or a Chi-square test. CAD: coronary artery dilatation, IVIG: intravenous immunoglobulin, AST: aspartate aminotransferase, ALT: alanine aminotransferase, CRP: C-reactive protein, Hct: hematocrit, WBC: white blood cell




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download