Abstract
Rheumatoid arthritis (RA) is frequently associated with various extra-joint complications. Although rare, thromboembolic complications are associated with high morbidity and mortality. We experienced a very rare case of nonbacterial thrombotic endocarditis (NBTE) and subsequent embolic stroke in a patient with RA. A 72-year-old male with a 15-year history of RA suddenly developed neurologic symptoms of vomiting and dizziness. Brain magnetic resonance imaging revealed recently developed multiple cerebellar and cerebral lacunar infarctions. Echocardiography showed a pulsating mitral valve vegetation involving the posterior cusp of the mitral valve leaflet, which was confirmed as NBTE. Immediate anti-coagulation therapy was started. The NBTE lesion disappeared in follow-up echocardiography after 4 weeks of anti-coagulation treatment.
Nonbacterial thrombotic endocarditis (NBTE), a very rare condition that refers to a spectrum of noninfectious endocarditis of the heart valves, is characterized by deposition of sterile platelet thrombi on the heart valve leaflets. NBTE is most commonly seen in advanced malignancy, hence, it is often an autopsy finding. While malignancies explain about 80% of NBTE cases, less common etiologies include inflammatory rheumatic diseases such as systemic lupus erythematosus, anti-phospholipid syndrome, rheumatoid arthritis (RA) and sepsis.1)
RA is a systemic autoimmune disease of unknown etiology characterized by chronic synovial inflammation of diarthrodial joints, worsening quality of life. Although RA patients have an increased risk of cardiovascular events such as myocardial infarction, stroke, cardiac death and thromboembolism compared with the general population,2)3) there has been no report of mitral valvular NBTE in a patient with RA. Thus, we presented a case of NBTE in a patient with a long history of RA.
A 72-year-old male patient was admitted for nausea and dizziness for 2 weeks. Clinical history showed that the patient had been diagnosed with seropositive RA 15 years prior. At that time, he had inflamed joints in both the shoulder, elbow, wrist, knee and foot. On plain X-rays, both metacarpophalangeal, right shoulder and knee joints showed multiple bone erosion and joint space narrowing. He had serum positivity of anti-cyclic citrullinated peptide antibody (11.6 U/mL) and high rheumatoid factor (278 IU/mL). Thus, he was diagnosed as RA according to the American College of Rheumatology 1987 revised RA classification criteria. Combination therapy of disease-modifying anti-rheumatic drug including methotrexate, sulfasalazine, bucillamine and low dose oral steroid was initiated to control his RA activity.
During the course of RA treatment, he underwent left total knee replacement surgery due to intractable pain and loss of function of his knee joint. Otherwise, his overall RA activity was stable without any aggravation. However, 2 weeks before his admission, he developed sudden onset headache, dizziness and nausea and was admitted to the rheumatology clinic in our hospital. He had not undergone any embolic event. On examination, his body temperature was 37.1°; and his blood pressure and heart rate were 140/80 mmHg and 77 beats/min, respectively. Respiration appeared normal, with a rate of 18 breaths/min.
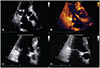
The initial laboratory tests revealed a white blood cell count of 7560/uL, hemoglobin concentration of 11.5 g/dL, platelet count of 367000/uL, erythrocyte sedimentation rate of 9 mm/hr, C-reactive protein of 0.40 mg/L, aspartate transaminase of 18 IU/L, alanine transaminase of 15 IU/L, lactate dehydrogenase of 72 IU/L, prothrombin time of 8.2 sec, activated partial thromboplastin time of 28.4 sec, the international normalized ratio of 0.74 and elevated D-dimer of 597 ng/mL. At the time of admission, he had been on hydroxychloroquine 200 mg/day, cylcosporin 50 mg/day and triamcinolone 4 mg/day. Anti-nuclear antibody was positive at 1:40. An elevated serum rheumatoid factor level of 119 IU/mL was still observed. Cardiac enzymes were not elevated. Chest X-ray showed no pathologic findings. Electrocardiogram showed normal sinus rhythm. Aerobic and anaerobic blood cultures revealed no growth of organisms. Hepatitis B surface antigen and Hepatitis C antibody were negative. Cold agglutinin test was negative. However, transthoracic echocardiography showed a mobile 0.3×2.3 cm-sized pulsating hyperechoic nodule involving the posterior mitral valve leaflet (Fig. 1). The nodule demonstrated to–and-fro motion and there was trivial mitral regurgitation. Other masses did not appear. Brain magnetic resonance imaging revealed recent multiple lacunar infarctions in the right posterior-inferior cerebellar arterial territory, right anterior-middle cerebral arterial border zone and left anterior cerebral arterial territory (Fig. 2). Anti-coagulation therapy with subcutaneous enoxaprin and warfarin was started, maintaining international normalized ratio at the target level of 3 during 4 weeks. We also used prophylactic third generation cephalosporin. Surgical intervention was considered to remove the vegetation. However, 4 weeks later, follow-up echocardiography showed no residual vegetation and the patient's initial neurologic deficit recovered. At the time of follow-up echocardiography, he had been on warfarin and his international normalized ratio was being maintained at the level of 3.13. Currently, follow up by the rheumatology clinic shows low RA disease activity.
Since the initial introduction of the term NBTE, there have been numerous reports on the relationship between NBTE and a variety of different inflammatory states, particularly malignancy.4) A definite diagnosis can be made pathologically by the demonstration of platelet thrombi on surgical specimens. However, because cardiac valvular tissue is not routinely available, clinicians rely on several clinical findings in the absence of microbiologic findings. Typically, the demonstration of valvular vegetations on echocardiography in the absence of infection provides strong evidence to confirm the diagnosis of NBTE.
RA is the most common systemic autoimmune disorder, characterized by chronic inflammation of synovial structures that leads to progressive joint destruction and disability. The relationship between RA and cardiovascular diseases is of particular interest because of the growing recognition of the contribution of chronic inflammation to the increased risk of cardiovascular events.5)6) In a meta-analysis, the mortality risk of ischemic heart disease and cerebrovascular accidents rose by 59% and 52%, respectively, in RA patients, as compared with the general population.7) Traditional individual cardiovascular risk factors alone, such as hypertension, diabetes, hypercholesterolemia and smoking,8) cannot entirely explain the higher risk. Evidence suggests that RA disease activity and medication used to dampen RA activity such as disease-modifying anti-rheumatic drug and biologic disease-modifying anti-rheumatic drug can also play a pivotal role in the development of cardiovascular risk.9)
The 3 previously reported cases10)11)12) of a cardiac valvular nodule in patients with RA are different from our case, in terms of histologic nature of the nodules. In these cases, the vegetative valvular nodules were rheumatoid nodules, confirmed by excisional biopsy.10)11) Of these, one case report described a complicated femoral artery embolic occlusion originating from a pedunculated mitral valvular rheumatoid nodule.12) However, the authors did not use the term NBTE because the cardiac valvular vegetation was inflammatory tissue and not a thrombus. In the study on surgical pathology of NBTE in 30 patients from 1985 to 2000 at the Mayo Clinic, 2 RA patients had NBTE.1) Their NBTE reportedly occurred in the aortic valve, which differed from the valvular location of our case.
The vegetation in NBTE consists of degenerating platelets interwoven with strands of fibrin, immune complexes and mononuclear cells. The vegetative mass varies in size from microscopic to large and aggressive. Compared to infective endocarditis, vegetations in NBTE are easily detached and cause extensive infarction. The pathogenesis of NBTE is unknown, but endothelial injury in a hypercoagulable state is thought to be essential for its development. Elevated circulating inflammatory cytokines such as tumor necrosis factor–α and interleukin-1 may also trigger vegetation formation. RA patients have a 1.5 to 6 times increased risk of venous thromboembolism, as compared with non-RA subjects,13)14) causing deep vein thrombosis and pulmonary thromboembolism. The major pro-inflammatory RA cytokines, including interleukin-6, interleukin -8 and tumor necrosis factor–α, are also thought to increase the risk of thrombosis in RA patients by activation of coagulation pathways or alteration of thrombotic tendency.15) Echocardiography is used to elucidate the presence of valvular vegetations. If transthoracic echocardiography is unrevealing, transesophageal echocardiography should be considered for suitable candidates. The major differential diagnosis is infective endocarditis. Culture with special attention to rule out infective endocarditis can provide distinction between these 2 entities.
Anticoagulation is the mainstay of therapy for patients with acute ischemic stroke with or without evidence of systemic emboli. If intracranial hemorrhage is ruled out with computed tomography, NBTE patients should be anticoagulated. Anticoagulation should be continued indefinitely regardless of underlying diseases, which should be treated when appropriate. In patients with systemic lupus erythematosus, NBTE does not correlate with disease activity.16) In the present RA case, arthritic activity was not higher around the time of the cardiovascular event. The patient had stable low disease activity. Discontinuation of anticoagulation is appropriate when bleeding complications occur; and can be individualized according to risk factors for recurrence and bleeding.17)
Although NBTE is a rare condition and the most prevalent endocarditis at autopsy, RA patients have an increased risk of cardiovascular events and thromboembolism. Consequently, all patients with RA require cardiac evaluation with echocardiography, if there are any abnormalities suggesting valvular heart disease independent of arthritic activity.
Figures and Tables
Fig. 1
Mitral valve vegetation. (A, B) Transthoracic echocardiography showing a 0.27×2.27 cm sized characteristic hyperechoic mass-like nodular lesion (NBTE, arrow) attached to the posterior cusp of the mitral valve. This lesion had a stem connecting the mass to the mitral valve, causing the lesion to show a to and fro motion in accordance with the cardiac cycle. (C, D) After 4 weeks of anticoagulation therapy, the NBTE lesion disappeared almost completely on echocardiography. NBTE: nonbacterial thrombotic endocarditis.
References
1. Eiken PW, Edwards WD, Tazelaar HD, McBane RD, Zehr KJ. Surgical pathology of nonbacterial thrombotic endocarditis in 30 patients, 1985-2000. Mayo Clin Proc. 2001; 76:1204–1212.
2. Meune C, Touzé E, Trinquart L, Allanore Y. Trends in cardiovascular mortality in patients with rheumatoid arthritis over 50 years: a systematic review and meta-analysis of cohort studies. Rheumatology (Oxford). 2009; 48:1309–1313.
3. Solomon DH, Karlson EW, Rimm EB, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003; 107:1303–1307.
4. el-Shami K, Griffiths E, Streiff M. Nonbacterial thrombotic endocarditis in cancer patients: pathogenesis, diagnosis, and treatment. Oncologist. 2007; 12:518–523.
5. Turiel M, Sitia S, Atzeni F, et al. The heart in rheumatoid arthritis. Autoimmun Rev. 2010; 9:414–418.
6. Wislowska M, Sypula S, Kowalik I. Echocardiographic findings, 24-hour electrocardiographic Holter monitoring in patients with rheumatoid arthritis according to Steinbrocker's criteria, functional index, value of Waaler-Rose titre and duration of disease. Clin Rheumatol. 1998; 17:369–377.
7. Aviña-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 2008; 59:1690–1697.
8. Baghdadi LR, Woodman RJ, Shanahan EM, Mangoni AA. The impact of traditional cardiovascular risk factors on cardiovascular outcomes in patients with rheumatoid arthritis: a systematic review and meta-analysis. PLoS One. 2015; 10:e0117952.
9. Kim SC, Solomon DH, Liu J, Franklin JM, Glynn RJ, Schneeweiss S. Risk of venous thromboembolism in patients with rheumatoid arthritis: initiating disease-modifying antirheumatic drugs. Am J Med. 2014; 128:539.e7–539.e17.
10. Chatzis A, Giannopoulos N, Baharakakis S, Saridakis N, Agapitos E, Stamatelopoulos S. Unusual cause of a stroke in a patient with seronegative rheumatoid arthritis. Cardiovasc Surg. 1999; 7:659–660.
11. Mounet F, Soula P, Concina P, Baradat G, Céréne A. [Heart valve diseases specific in rheumatoid polyarthritis. Apropos of 2 cases]. Arch Mal Coeur Vaiss. 1997; 90:987–989.
12. Kang H, Baron M. Embolic complications of a mitral valve rheumatoid nodule. J Rheumatol. 2004; 31:1001–1003.
13. Choi HK, Rho YH, Zhu Y, Cea-Soriano L, Aviña-Zubieta JA, Zhang Y. The risk of pulmonary embolism and deep vein thrombosis in rheumatoid arthritis: a UK population-based outpatient cohort study. Ann Rheum Dis. 2013; 72:1182–1187.
14. Bacani AK, Gabriel SE, Crowson CS, Heit JA, Matteson EL. Noncardiac vascular disease in rheumatoid arthritis: increase in venous thromboembolic events? Arthritis Rheum. 2012; 64:53–61.
15. van der Poll T, Büller HR, ten Cate H, et al. Activation of coagulation after administration of tumor necrosis factor to normal subjects. N Engl J Med. 1990; 322:1622–1627.
16. Ong ML, Veerapen K, Chambers JB, Lim MN, Manivasagar M, Wang F. Cardiac abnormalities in systemic lupus erythematosus: prevalence and relationship to disease activity. Int J Cardiol. 1992; 34:69–74.
17. Mazokopakis EE, Syros PK, Starakis IK. Nonbacterial thrombotic endocarditis (marantic endocarditis) in cancer patients. Cardiovasc Hematol Disord Drug Targets. 2010; 10:84–86.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download