This article has been
cited by other articles in ScienceCentral.
Abstract
Abdominal wall hematoma is a rare but potentially serious vascular complication that may develop after coronary angiographic procedures. In particular, an oblique muscle hematoma caused by an injury of the circumflex iliac artery is very rare, yet can be managed by conservative treatment including hydration and transfusion. However, when active bleeding continues, angiographic embolization or surgery might be needed. In this study, we report an uncommon case of injury to the circumflex iliac artery by an inappropriate introduction of the hydrophilic guidewire during the performance of a percutaneous coronary intervention.
Keywords: Percutaneous coronary intervention, Hematoma, Femoral artery
Introduction
Vascular complications are the most frequent adverse outcomes associated with percutaneous coronary intervention (PCI) via the femoral artery. They contribute to in-hospital morbidity, mortality, and costs; and furthermore, are associated with increased long-term risk of myocardial infarction and death. Vascular complications include bleeding, pseudoaneurysm formation, hematoma, arterio-venous fistula, retroperitoneal bleeding, and other femoral arterial injuries requiring procedural or surgical intervention. Abdominal wall hematoma is a rare condition that can give rise to an acute abdomen.
In this report, we describe a case of a 73-year-old woman who developed an abdominal wall hematoma, which is a rare but serious complication of a PCI via the femoral approach.
Case
A 73-year-old woman, with a past medical history of hypertension, diabetes mellitus for 40 years, and chronic kidney disease stage 5, because of diabetic nephropathy, presented to the emergency department with dyspnea (NYHA IV). The patient had the following vital signs: blood pressure: 155/63 mmHg; pulse: 93 beats per minute; respiratory rate: 28 breaths per minute; and her body temperature was 36.0℃. On physical examination, the patient had no notable findings, other than bilateral expiratory rales and neck vein distention. No heart murmurs or pericardial friction rub were heard. An electrocardiogram was within normal limits. A chest X-ray revealed marked cardiomegaly and pulmonary edema. Laboratory tests showed the following: white blood cell count: 10300/mm3, hemoglobin: 8.3 g/dL, platelet count: 250000/mm3, blood urea nitrogen: 63 mg/dL, creatinine: 4.4 mg/dL, brain natriuretic peptide: 483 pg/mL, creatine kinase isoenzyme MB: 2.0 ng/mL, and troponin-I: 0.10 ng/mL. The patient received emergency hemodialysis because of pulmonary edema refractory to medical therapy, including high-dose furosemide infusion. After stabilizing the patient’s volume status by regular hemodialysis, she received coronary angiography for the evaluation of ischemic heart disease on the 19th day of admission. The patient took aspirin (300 mg) and clopidogrel (300 mg) loading dose 12 hours before the procedure.
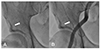
Exploration was performed via the right femoral artery. When the 0.025-inch straight guidewire (45 cm, Terumo, Tokyo, Japan) for a 4 French sheath was advanced, some resistance was felt. We then checked the fluoroscopy and found that the guidewire was introduced into the circumflex iliac artery (
Fig. 1A). We promptly pulled the guidewire back from the circumflex iliac artery and advanced it into the abdominal aorta under fluoroscopic guidance. After the 4 French sheath was inserted, we checked the femoral angiography. Neither perforation nor dissection from the circumflex iliac artery was shown (
Fig. 1B). The external iliac artery and circumflex iliac arteries were intact, there were no changes in the patient’s vital signs, and no abdominal symptoms were found, therefore, we continued the coronary angiography without incident.
Fig. 1
Right femoral arteriography. (A) Hydrophilic guidewire is unintentionally introduced into the circumflex iliac artery (arrow). (B) Femoral and iliac arteriography after withdrawing the guidewire. The circumflex iliac artery is intact and there is no evidence of perforation (arrow).
Coronary angiography revealed significant stenosis in the middle and distal segments of the right coronary artery and the middle segment of the left anterior descending artery. After 7000 IU of heparin was administered intravenously, we performed a PCI at the middle and distal right coronary artery and the mid-left anterior descending artery with an activated clotting time (ACT) of more than 300 seconds, without any immediate complications. Because the ACT remained prolonged, the sheath removal was performed 12 hours after PCI without any acute complications. Fourteen hours after PCI, the patient complained of a right abdominal pain, with mild swelling of her right abdomen. Her blood pressure abruptly dropped to 80/40 mmHg. We started hydration with normal saline and performed a transfusion of 2 pints of packed red blood cell because of an abrupt drop of hemoglobin from 9.3 to 7.3 g/dL over 12 hours. After hydration and transfusion, her blood pressure returned to 110/70 mmHg. The sheath site was clear with no evidence of bleeding, hence, we performed an abdominal computed tomography angiography to identify other causes of the bleeding, which revealed a right lateral abdominal wall hematoma about 5 cm in diameter (
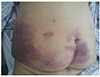
Fig. 2). The right circumflex iliac artery was located in the hematoma, but there was no evidence of active extravasation of contrast media. The patient’s vital signs remained stable after hydration and transfusion, so we decided to closely observe her. However, the patient still complained of abdominal pain and ecchymotic patches appeared on her abdomen 3 days after the procedure (
Fig. 3). Next, the patient underwent intermittent hemodialysis with minimal use of heparin and had her hemoglobin checked daily. After 5 days, her abdominal pain resolved and the ecchymosis gradually improved. There was no evidence of further hemoglobin changes. The patient was discharged uneventfully after 26 days of hospitalization (7 days after PCI). She was discharged on aspirin (100 mg), clopidogrel (75 mg), rosuvastatin (10 mg), losartan (50 mg), and carvedilol (6.25 mg). No further complications were evident, after one year of follow-up.
Fig. 2
Abdomen computed tomography angiography. Transverse (A) and coronal (B) section show right-sided abdominal hematoma and circumflex iliac artery (arrows). There is no evidence of extravasation of contrast media.

Fig. 3
Photograph of the patient with abdominal wall hematoma at 3 days after the procedure. Ecchymotic patches appears on the patient's lower abdomen, especially the right side.
Discussion
Abdominal wall hematoma is a rare but potentially serious vascular complication that may develop after cardiac and peripheral angiographic procedures.
1) Predisposing factors include increased sheath size, repeat or multiple punctures of the artery, concomitant use of anticoagulants, advanced age, being female, and hypertension.
2)3) There are two types of abdominal wall hematomas: spontaneous and iatrogenic. Iatrogenic hematomas often occur when the circumflex iliac artery is perforated during the insertion of the guidewires, although this is a rare complication.
4) These hematomas are distinguished from retroperitoneal hematomas, which occur when a physician unintentionally punctures the inferior epigastric artery or cannulates the femoral artery too superiorly. There have been cases of spontaneous abdominal wall hematomas in patients with some predisposing factors, although the incidence is very low.
5)6)
The common symptoms of abdominal wall hematoma are the sudden onset of abdominal pain and swelling, which usually occurs several hours after the procedure. Because of its rarity, abdominal wall hematoma can be mistaken for several common acute abdominal conditions, such as appendicitis, sigmoid diverticulitis, perforated ulcers, ovarian cyst torsion, tumors, and incarcerated inguinal hernias.
7) Diagnosis can be made clinically by the appearance of an obvious swelling or bruising, or may include non-specific findings, such as anemia and fever. Imaging studies help to confirm the diagnosis and exclude intra-abdominal hemorrhage.
8) Contrast-enhanced CT can detect and evaluate active bleeding from a rupture site. Even in patients without contrast extravasation at the bleeding site as observed on a CT, angiography could be a useful imaging technique to identify an active bleeding point.
9) Conservative management, including bed rest and analgesics, is appropriate for most stable patients. However, when a patient has evidence of sustained bleeding, angiographic interventions or surgery should be considered.
10)
In order to prevent vascular complications, such as abdominal wall hematomas or pseudoaneurysms, puncturing the femoral artery under fluoroscopy guidance may be considered.
11) After the artery has been successfully punctured, the guidewire must be smoothly advanced through the cannula. The cornerstone of safe sheath engagement is to stop when resistance is encountered during the insertion of the guidewire. Difficulty in advancing the guidewire may occur when the wire enters a small branch vessel. After confirming the location of a wire by fluoroscopy, one can then retract the wire, spin it gently, and try advancing it again. In this situation, one must consider angiography to assess for the occurrence of complications, such as vessel perforation and dissection. If complications are evident, then bleeding control should be prioritized. When vascular complications are suspected, PCI should be delayed with a discontinuation of antithrombotic agents, as well as close observation of the patient.
Because hydrophilic guidewires tend to unintentionally engage small vessels, replacing the straight hydrophilic guidewire with a J-tipped wire can be one strategy for preventing the perforation of small vessels. In general, it cannot be overemphasized that it is important to closely observe patients who have undergone PCI, especially with those with predisposing factors for bleeding, even without any evidence of immediate vascular complications.
In the present case, there was an unusual development of an abdominal wall hematoma after a PCI, in the absence of immediate vascular complications. Thus, we suggest that the following may be causative factors of a delayed abdominal wall hematoma following a PCI: injury to the circumflex artery during guidewire insertion and the subsequent prolonged ACT associated with the use of periprocedural heparin in patients with chronic kidney disease.
This case reminds medical professionals of the importance of close observation and proper evaluation of complications after percutaneous coronary intervention, even if the rates of these complications are very low. Fluoroscopy-guided femoral artery puncture and guidewire insertion may reduce the rate PCI-related vascular complications. When vascular complications are suspected during the procedure, a staged PCI should be considered to prevent periprocedural vascular complications and ensure a patient’s safety. With more coronary angiographic procedures being performed, it is important for clinicians to consider the possibility of abdominal wall hematoma in this situation, especially when the patient has many predisposing factors. Moreover, it is important that this complication is promptly recognized and managed.

