Abstract
Background and Objectives
Subjects and Methods
Results
Figures and Tables
Fig. 1
In baseline echocardiography, postoperative echocardiography at 7 days follow-up and postoperative echocardiography at least 3 months follow-up analysis, LVEDD decreased from the baseline echocardiography toward postoperative echocardiography at least 3 months follow-up. And patients with LV remodeling group were larger LVEDD than patients with no-remodeling group. LVEDD: left ventricular end-diastolic dimension, LV: left ventricular.

Fig. 2
It was shown the change of (A) endocardium GLS, (B) mid-layer GLS, (C) epicardium GLS and (D) LVEF in the measuring time. GLS: global longitudinal strain, LVEF: left ventricular ejection fraction.

Fig. 3
Scatter diagram with Pearson's correlation between baseline mid-layer GLS by 2D MSTE and (A) postoperative LVEDD, (B) postoperative LVEF. GLS: global longitudinal strain, 2D MSTE: two-dimensional multilayer speckle-tracking echocardiography, LVEDD: left ventricular end-diastolic dimension, LVEF: left ventricular ejection fraction.

Fig. 4
2D mid-layer GLS was the most useful cutoff value to severe MR with normal LVEF by receiver-operating characteristic curve analysis. GLS: global longitudinal strain, MV: mitral valve repair, LVEF: left ventricular ejection fraction.
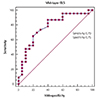
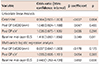
Table 1
Baseline Characteristics of Patients with non-remodeling group and remodeling group

Data are listed as numbers (percentage of group) and mean value. The p denotes statistical significance comparing the non-remodeling group and remodeling group. *p<0.05 by student t-test (continuous variable), Chi-square (categorical variables). BSA: body surface area, SBP: systolic blood pressure, DBP: diastolic blood pressure, HR: heart rate, NT-proBNP: N-terminal of the prohormone brain natriuretic peptide, HTN: hypertension, DM: diabetes mellitus, CKD: chronic kidney disease, ACEi: angiotensin-converting enzyme inhibitors, ARB: angiotensin receptor blocker, CCB: calcium channel blocker, MVR: mitral valve replacement, MVP: mitral valve plasty
Table 2
Baseline, post OP 7days and post OP 3 month echocardiographic parameters of patients with non-remodeling group and remodeling group

Data are listed as numbers (percentage of group) and mean value. The p denotes statistical significance comparing the non-remodeling group and remodeling group. *p<0.05 by student t-test (continuous variable), Chi-square (categorical variables). LVEDD: left ventricular end-diastolic dimension, LVESD: left ventricular end-systolic dimension, IVSd: diastolic interventricular septum thickness, LVPWd: diastolic left ventricular posterior wall thickness, LVMI: left ventricular mass index, LAVI: left atrial volume index, LVEF: left ventricular ejection fraction, RVSP: right ventricular systolic pressure, GLS: global longitudinal strain
Table 3
Baseline LVEF, midGLS, at least 3 months follow-up LVEF, and at least 3 months follow-up midGLS in MV repair and MVR groups

Data are listed as mean value. The pdenotes statistical significance comparing the non-remodeling group and remodeling group. p<0.05 by student t-test (continuous variable). LVEF: left ventricular ejection fraction, GLS: global longitudinal strain, MV: mitral valve repair, MVR: mitral valve replacement
Table 4
Univariate linear and multivariate logistic regression analysis for determinants of postoperative LV remodeling




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download