Abstract
Background and Objectives
Subjects and Methods
Results
Figures and Tables
Fig. 1
Illustration of the location of the right vagus nerve and direct vagal stimulation. (A) The right vagus nerve crosses anterior to the right subclavian artery, runs posterior to the SVC, and contributes to the cardiac, pulmonary, and esophageal plexuses. (B) A steerable quadri-polar catheter was placed in the SVC, just above the suture line, for the stimulation of the cardiac branches of the recipient's right vagus nerve. SVC: superior vena cava, IVC: inferior vena cava.
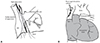
Fig. 2
Scatterplots showing the significant positive correlations between the time after HTx and heart rate variability parameters, including LF (A), HF (B), ASDNN (C) and RMSSD (D). The vertical dot lines divide the subjects into early (0.5-1 year), intermediate (1-2 years) and late (>2 years) groups after HTx. HTx: heart transplantation, LF: low-frequency component, HF: high-frequency component, ASDNN: average of all 5-minute standard deviations of the NN interval, RMSSD: square root of the difference between consecutive RR-intervals.

Fig. 3
Incremental changes in the HRV analysis of parasympathetic parameters. RMSSD (A) and HF (B) were significantly higher during the late period (>2 years) compared with the early period (0.5-1 year) after HTx. *p<0.017 indicates the presence of a statistically significant difference between two groups in the post-hoc analysis. HRV: heart rate variability, RMSSD: square root of the difference between consecutive RR-intervals, HF: high-frequency component, HTx: heart transplantation.

Fig. 4
Serial assessment of HRV at 6 and 12 months after heart transplantation. (A) LF, (B) HF, (C) ASDNN, and (D) RMSSD. There were no statistically significant changes between 6 and 12 months, likely because of the small sample size. However, modest incremental changes were observed (p<0.05 indicates a statistically significant result). HRV: heart rate variability, LF: low-frequency component, HF: high-frequency component, ASDNN: average of all 5-minute standard deviations of the NN interval, RMSSD: square root of the difference between consecutive RR-intervals.

Table 1
Baseline characteristics of the study participants

Table 2
Comparisons of heart rate variability values with time after heart transplantation

Values are mean±standard deviation. p for trend was from the Johckheere-Terpstra test among 3 groups. HTx: heart transplantation, HR: heart rate, SDANN: standard deviation of the mean of RR intervals taken in 5-minutes segments, SDNN: standard deviation of the mean of all RR intervals, ASDNN: average of all 5-minute standard deviations of NN interval, pNN50: percentage of differences between successive normal RR-interval>50 msec, RMSSD: square root of the difference between consecutive RR intervals, LF: low-frequency component, HF: high-frequency component Table 3. Holter parameters (24-hour) and the results for direct vagal stimulation, at 6 and 12 months
Table 3
Holter parameters (24-hour) and the results for direct vagal stimulation, at 6 and 12 months





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download