Abstract
Background and Objectives
The number of patients with cardiac implantable electronic devices needing lead extraction is increasing for various reasons, including infections, vascular obstruction, and lead failure. We report our experience with transvenous extraction of pacemaker and defibrillator leads via the inferior approach of using a gooseneck snare as a first-line therapy and compare extraction using a gooseneck snare with extraction using simple manual traction.
Subjects and Methods
The study included 23 consecutive patients (43 leads) who underwent transvenous lead extraction using a gooseneck snare (group A) and 10 consecutive patients (17 leads) who underwent lead extraction using simple manual traction (group B). Patient characteristics, indications, and outcomes were analyzed and compared between the groups.
Results
The dwelling time of the leads was longer in group A (median, 121) than in group B (median, 56; p=0.000). No differences were noted in the overall procedural success rate (69.6% vs. 70%), clinical procedural success rate (82.6% vs. 90%), and lead clinical success rate (86% vs. 94.1%) between the groups. The procedural success rates according to lead type were 89.2% and 100% for pacing leads and 66.7% and 83.3% for defibrillator leads in groups A and B, respectively. Major complications were noted in 3 (mortality in 1) patients in group A and 2 patients in group B.
The number of cardiac implantable electronic devices (CIEDs), including permanent pacemakers and implantable cardioverter defibrillators (ICDs), has been increasing globally.1) With the increase in the number of patients with CIEDs and major incidence of comorbidities, the rate of CIED-related infection has risen markedly, resulting in a high number of complete CIED removals.2)
Extraction of permanent pacemaker leads or defibrillator leads is a challenging procedure. The techniques and tools for transvenous lead extraction have undergone substantial improvement over the past several decades. The use of locking stylets and mechanical and powered sheaths (laser or electrosurgical sheaths) has significantly improved the success rate.3)4)5)6) However, because of the unavailability and significant financial expense of the current standard tools for lead extraction, including locking stylets and mechanical, laser, and mechanical dilator sheaths in Korea, alternative lead extraction techniques using more readily available tools are urgently needed.
The aim of the present study was to report our experience of the indications, success rates, and complications of transvenous extraction of pacemaker and defibrillator leads via an inferior approach using a gooseneck snare with or without an ablation catheter as first-line therapy and compare extraction using a gooseneck snare with extraction using simple manual traction.
A total of 57 patients with CIEDs underwent transvenous or surgical lead extraction at Asan Medical Center between September 2008 and May 2015. Among these patients, those with lead dwelling time of less than 1 year (n=15) and those in whom leads were completely extracted with open thoracotomy (n=9) were excluded. So, 33 consecutive patients who underwent transvenous lead extraction were enrolled in the present study. In all patients, simple manual traction via lead entry site was attempted first. If the simple manual traction failed, an inferior approach using a gooseneck snare was employed. Thus, the patients were divided into group A (leads were extracted using a gooseneck snare; 23 patients, 43 leads) and group B (leads were extracted using only simple manual traction; 10 patients, 17 leads). Patient characteristics, lead and device characteristics, indications for extraction, and outcomes were retrospectively analyzed. This study was approved by the institutional review board of Asan Medical Center, and all patients provided informed consent.
The indications for transvenous lead extraction were determined according to the Heart Rhythm Society (HRS)/American Heart Association (AHA) 2009 consensus document.7)
A total of 11 procedures were performed. Of these 11 procedures, 9 were performed in a dedicated electrophysiology laboratory under local anesthesia and conscious sedation, with on-site cardiac surgery back-up available to intervene in the event of an emergency, and 2 were performed in an operation room under general anesthesia. All procedures were performed with cutaneous pads for defibrillation, transvenous temporary pacing, invasive arterial blood pressure monitoring, electrocardiography monitoring, and pulse oximetry monitoring. After removal of the device and dissection of fibrous tissue around the lead, simple traction of the lead was performed following insertion of a non-locking stylet and retrieval of screws until separation of the lead from the myocardium and venous system was accomplished (Fig. 1A).
A total of 36 procedures were performed. Of these 36 procedures, 30 were performed in a dedicated electrophysiology laboratory and 6 were performed in an operation room under general anesthesia. The procedural preparation was the same as that for simple manual traction.
The extraction technique was performed as follows: a commercially available steerable ablation catheter or Amplatz GooseNeck snare (Microvena Corporation, White Bear Lake, MN, USA) were inserted via 10- or 11-F and 8-F femoral vein sheathes, respectively. In the right atrium, the flexed ablation catheter was rotated in alternate clockwise and counter-clockwise directions to catch the lead. Once the lead was caught, the gooseneck snare was used to grasp the tip of the ablation catheter (Figs. 1B and 2A), and then the snare was closed and locked. Intermittent traction and release of both the ablation catheter and the gooseneck snare were applied to keep the lead tense in order to reduce contact with the myocardial walls and avoid myocardial wall damage (Figs. 1C-D and 2B). When the distal tip of the lead was freed from the myocardium, simple traction of the lead body from the entry site was attempted (Figs. 1E, 1F and 2C). If resistance was noted, the lead was cut at the entry site, and the free-floating lead fragment was re-grasped using the gooseneck snare (Fig. 2D and 2E). The grasped lead was gently pulled out of the vessel and into the sheath (Fig. 2F). Hemostasis of the femoral access site was achieved with manual compression only.
Outcome definitions have been previously reported in the HRS/AHA 2009 consensus document.7) Complete procedural success is defined as the removal of all targeted leads and all lead material from the vascular space, with the absence of any permanently disabling complication or procedure-related death. Clinical success is defined as the removal of all targeted leads and lead material from the vascular space or retention of a small portion of the lead that did not negatively impact the goals of the procedure. Examples include the tip of the lead or a small part of the lead (conductor coil, insulation, or the two combined) when the residual part did not increase the risk of perforation, embolic events, perpetuation of infection, or any other undesirable outcome. Lead clinical success is defined as number of leads removed with clinical success/total number of leads attempted. Failure is defined as the inability to achieve either complete procedural or clinical success, or the development of any permanently disabling complication or procedure-related death. The definitions of major and minor complications related to the procedure are presented in the HRS/AHA 2009 consensus document.7) Major complications are defined as those that were life-threatening or that resulted in death. Minor complications are defined as those related to the procedure which required medical intervention or additional procedural intervention.
Extraction procedure time is the time interval from skin incision to the extraction of the last lead. The lead extraction time is the time interval from insertion of the snare or ablation catheter via the femoral vein to extraction of the first single target lead and the time interval from extraction of the preceding single target lead to extraction of the next single target lead.
Continuous variables are reported as means (normally distributed) or medians (non-normally distributed), while categorical variables are reported as numbers (percentages). Between-group comparisons were made using a t-test for normally distributed continuous variables; otherwise, the Mann-Whitney U or Wilcoxon test was used. The chi-square test was used for categorical variables. Differences in the mean values between the 2 groups were compared using the chi-square test and paired t-test. A p<0.05 was considered statistically significant in all analyses. All statistical analyses were performed using PASW statistics version 18.0.0 (SPSS Inc., Chicago, IL, USA).
The mean age of the enrolled patients was 58.1±14.1 years (range, 26–75 years). There were 23 male and 10 female patients. The indications for lead removal included infection (n=16), lead malfunction (n=16), prevention of venous occlusion (n=1), and patient's discretion (n=2). A total of 48 pacemaker leads and 12 defibrillator leads were extracted. The fixation mechanisms were passive in 43 leads and active in 17 leads. The median dwelling time of the leads was 106 months (interquartile range, 57–152 months), and the median dwelling time of the leads was longer in group A (median, 121; interquartile range, 83–192 months) than in group B (median, 56; interquartile range, 35–95 months; p=0.000) (Table 1).
In group A, complete removal of the leads was achieved in 16 patients, with a complete procedural success rate of 69.6% (Table 2). In patients 6, 14, and 20, extraction of the pacing and defibrillator leads was abandoned. In patient #23, the remnant pacing lead was extracted using a surgical approach without thoracotomy. Thus, the clinical success rate was 82.6% (19/23).
In group B, the complete procedural success rate was 70% (Tables 3 and 4). In 2 patients, small tip portions of the ventricular pacemaker lead permanently remained after the procedure, without any undesirable outcomes. In patient #6, extraction of the defibrillator lead was abandoned and a new defibrillator lead was implanted. Thus, the clinical success rate was 90% (9/10).
Lead extraction times were measured in a total of 51 lead extractions for 60 leads. The mean lead extraction time was significantly lower in group A (14.2±21.4 minutes) than in group B (38.5±45.2 minutes; p=0.035). Additionally, the mean lead extraction time was significantly lower for atrial leads (14.5±19.8 minutes) than for ventricular leads (38.9±46.1 minutes; p=0.031). However, the mean lead extraction time did not differ between active fixation leads (26.5±40.0 minutes) and passive fixation leads (32.73±36.6 minutes; p=0.609).
The clinical success rate of pacemaker leads was 89.2% in group A and 100% in group B (p=0.26). Additionally, the clinical success rate of defibrillator leads was 66.7% in group A and 83.3% in group B (p=0.51). Moreover, the clinical success rate of infected leads was 88.9% in group A and 100% in group B (p=0.23) (Table 4).
Major complications occurred in 3 patients in group A and 2 patients in group B (13% and 20%, respectively). There was no immediate mortality or necessity for open-heart surgery in either group. In group A, patient #5 experienced thrombosis from the superior vena cava (SVC) to the left subclavian vein due to a residual SVC coil. Balloon angioplasty and removal of the residual coil restored blood flow. Additionally, patient #14 experienced progressive heart failure and shock after failed extraction of defibrillator leads, and underwent medical treatment and extracorporeal membrane oxygenation and, finally, heart transplantation 5 months after failed lead extraction. Patient #20 died because of an uncontrolled Candida infection related to central venous catheter use 30 days after attempted extraction.
In group B, patient #6 experienced pericardial effusion directly related to implantation of a new defibrillator lead, which required pericardiocentesis. Additionally, patient #10 experienced a hematoma and skin defect at the wound site, which required bleeding control and wound revision.
Among 10 patients with ICDs, 6 underwent re-implantation of ICDs within one month, while re-implantation of ICDs in the other four patients was deferred for several reasons (recovery from heart failure, no ventricular arrhythmia after ICD implantation, patient's refusal).
Among 22 patients with pacemakers, 14 underwent re-implantation of pacemakers in the same hospitalization period. The remaining patients did not undergo re-implantation because no definite indication for pacemaker implantation was noted after lead extraction.
In patients 6 and 14, atrial leads could not be extracted owing to tight adherence of the pacemaker leads to the SVC. In patient #14, SVC coils of 2 defibrillator leads tightly adhered to the SVC and subclavian vein, rendering extraction impossible. In patients 20 and 21, a free-floating pacing lead inside the tricuspid valve and a defibrillator lead, which was fractured during traction via the inferior approach, could not be grasped using the snare (Fig. 3).
The present study found that (1) the transfemoral approach was effective as a primary approach for the removal of pacing leads; (2) there was no cardiac tamponade, hemothorax, emergency cardiac operation, or mortality related to the procedure; and (3) the procedural success rate was lower for defibrillator leads than for pacing leads.
Even though the indwelling time of leads was longer in patients who underwent extraction using a gooseneck snare than in patients who underwent extraction using simple manual traction, there was no difference in the complete procedural success rate (69.6% vs. 70%), clinical procedural success rate (82.6% vs. 90%), and lead clinical success rate (86% vs. 94.1%) between patients who underwent extraction using a gooseneck snare and patients who underwent extraction using simple manual traction.
Transvenous extraction of leads can be performed either by a superior or inferior approach.3)8) The superior approach can be performed with simple traction, using a locking stylet or traction and countertraction using a mechanical sheath, locking stylet, mechanical dilator sheath, or laser sheath. The superior approach via the jugular vein using locking stylets and sheaths has favorable outcomes. These specialized tools have improved the success rate significantly. However, some of these tools (locking stylet, mechanical sheath, and laser sheath) are unavailable in developing countries and the tools are currently unavailable and not reimbursed by medical insurance in Korea.
The technique of intravascular removal of a foreign body was developed by Dotter et al.9) in 1971. The transfemoral approach is versatile and can be used for percutaneous retrieval of cardiac leads, indwelling catheters, fragments of catheter tubing or wire guides, and other foreign objects.9) Lead extraction via the inferior approach is the only interventional method for free-floating leads, as the proximal end of the lead cannot be approached at the generator pocket, and this approach is essential for pulling the lead from the SVC to the right atrium in the process of transjugular lead extraction.10) Traditionally, transfemoral extraction requires a 16-F (inner diameter) sheath with a hemostatic valve (Byrd Workstation, Cook Medical, Bloomington, IN, USA), which is inserted through the femoral vein. To grasp and extract the lead from the heart, a deflecting wire guide and a Needle's Eye snare (Cook Medical, Bloomington, IN, USA) are commonly used. In place of a deflecting wire, a deflectable ablation catheter and helical basket retriever (Dotter basket; Cook Medical, Bloomington, IN, USA) can be used for improved flexibility and steerability.11)
Lead extraction via the femoral approach using either a snare or Needle's eye snare has been reported to have a success rate of 87.2–95%, with variable complications.12)13) As mentioned earlier, advanced tools for femoral extraction are unavailable; therefore, modified femoral lead extraction was performed with an ablation catheter and gooseneck snare in the present study as an alternative to a deflecting wire and snare.14) In our study, 33 of 37 pacing leads were extracted successfully without major complications. A high success rate was obtained with transfemoral lead extraction, and there were no major complications, including cardiac tamponade, hemothorax, emergency operation, or mortality, directly related to the procedure. Vascular tear that may occur with a mechanical sheath can be avoided by using an inferior approach.
Defibrillator leads are prone to failure from conductor facture15) or insulation damage.16)17) Given the risks of high-voltage failure and sensing failure, replacement of the defibrillator lead is recommended with or without extraction of the advisory lead. Although a defibrillator lead can be extracted with simple traction, in more than 50% of cases a powered countertraction sheath is needed and an additional femoral approach may be required because of the high frequency of lead fracture.16)17)18)
Despite improvements in extraction techniques, lead extraction is still associated with a low but significant mortality rate.19)20)
In our study, 1 patient (#14, group B) with 2 defibrillator leads underwent 2 lead extraction procedures with failure after ICD recall. The patient underwent heart transplantation eventually. However, the defibrillator leads could not be removed during surgery. The coils of defibrillator leads have been shown to induce extensive growth of scar tissue, which surrounds and entraps the leads and requires complex extraction procedures.21)22) Areas of adherence of defibrillator leads were identified in the subclavian vein (78%), innominate vein (65%), SVC (66%), and heart (73%).23) Dwelling time, passive fixation, and dual-coil lead design have been shown to be independently associated with adherence.23) In patient #14, the long dwelling time and presence of 2 dual coil defibrillator leads contributed to the development of severe fibrosis and adherence to vasculature, preventing lead extraction even during surgery. Given the high adherence related to the dwelling time and dual-coil lead design, early lead extraction rather than lead reinsertion at the time of lead malfunction and use of a single coil lead might have improved the clinical outcome.
A laser sheath has been shown to improve the outcome of lead extraction of pacemaker or cardioverter-defibrillator leads.5) However, a randomized clinical trial reported no difference in the success rate between a laser sheath and the femoral approach.24) This result, together with the high success rate of the femoral approach,12)13)25) suggests that the femoral approach should be a primary method for lead extraction of chronic CIED leads not removable with simple traction in underdeveloped or developing countries where a laser sheath is unavailable.
The present study has several limitations. First, this was a single-center retrospective study. However, this is the first study to report femoral extraction of pacing and defibrillator leads as a primary approach and analyzed the largest number of Asian patients of any study so far. Second, availability and insurance coverage of locking stylets and sheaths might decrease the need for the transfemoral approach. However, the transfemoral approach would remain the only method for the extraction of a free-floating lead. Additionally, easy pullback of the lead binding in the SVC is a useful step in the process of complex lead extraction. Third, the use of the Byrd Workstation and a deflecting guidewire might improve the success rate, especially when using traction and countertraction. However, in a study by de Bie et al.25) selective use of femoral lead extraction without the Byrd Workstation was successful in 93.5% of cases. The superiority of the Byrd Workstation over a snare requires further investigation. Fourth, there were 4 cases of unsuccessful lead extraction. The use of a combined superior approach using a locking stylet and mechanical sheath might improve clinical outcomes. Fifth, as an SVC coil is a risk factor for difficulty in lead extraction26) and no differences are present in the clinical outcomes between a dual-coil and single-coil defibrillator lead,27)28) the preferential use of a single-coil defibrillator lead will allow for easy extraction.
Simple manual traction was safe and effective for the extraction of leads with a short dwelling time and infected leads, while the transfemoral approach using a gooseneck snare was safe and effective for the extraction of pacing leads with a long dwelling time. However, the clinical success rate is lower for defibrillator leads than for pacing leads. Therefore, the development of advanced extraction tools is an area of profound interest, especially for cases of defibrillator leads.
Figures and Tables
Fig. 1
Extraction of dual-chamber defibrillator leads. (A) The active fixation lead is removed by simple traction. (B) A closed loop is formed with the snare (white arrowhead) and ablation catheter (black arrowhead) capturing target ICD lead. (C) Downward traction of the snare and ablation catheter complex is performed gently. Repeated traction and release are required for complete removal. (D) The tip of the ICD lead is detached from the RV apex. The SVC coil is already moved into the subclavian vein with traction. (E) The entire ICD lead is removed by manual traction via the entry site. (F) Fluoroscopy shows absence of residual lead material. ICD: implantable cardioverter defibrillator, RV: right ventricle, SVC: superior vena cava.
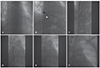
Fig. 2
Extraction of dual-chamber pacemaker leads. (A) The tip of ablation catheter is bended to anchor the right atrial lead. (B) A closed loop capturing target lead is formed with the snare and ablation catheter. (C) The right atrial lead is detached from the insertion site by gentle traction. (D) Manual removal of the detached right atrial lead via the entry site is impossible, probably due to venous occlusion. (E) The tip of the right atrial lead is grasped by the snare. (F) The lead is cut at the entry site. The proximal fragment was removed via entry site, and the distal fragment was removed easily via the femoral vein without residual lead material.
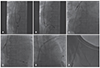
Fig. 3
Right anterior oblique views of the leads that could not be extracted via the femoral approach. (A) A remnant pacing lead in the right ventricle after surgical extraction could not be extracted owing to failure to capture the remnant lead. (B) A remnant defibrillator lead that was fractured during traction from below could not be removed owing to failure to capture the remnant lead.
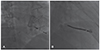
Table 1
Baseline characteristics of the study patients
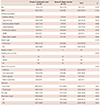
Table 2
Procedural results in patients who underwent extraction using a gooseneck snare (group A)

*Two procedures for extraction of the same lead.†,‡,§ RA lead was extracted with simple traction. RA: right atrium, P: passive fixation, CR: complete removal, RV: right ventricle, A: active fixation, ICD: implantable cardioverter-defibrillator, CRT-D: cardiac resynchronization therapy with a defibrillator, IR: incomplete removal
Table 3
Procedural results in patients who underwent extraction using simple manual traction (group B)

Table 4
Procedural outcome and complication after lead extraction in groups A and B

References
1. Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009--a World Society of Arrhythmia's project. Pacing Clin Electrophysiol. 2011; 34:1013–1027.
2. Greenspon AJ, Patel JD, Lau E, et al. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States: 1993 to 2008. J Am Coll Cardiol. 2011; 58:1001–1006.
3. Byrd CL, Schwartz SJ, Hedin NB, Goode LB, Fearnot NE, Smith HJ. Intravascular lead extraction using locking stylets and sheaths. Pacing Clin Electrophysiol. 1990; 13(12 Pt 2):1871–1875.
4. Smith HJ, Fearnot NE, Byrd CL, Wilkoff BL, Love CJ, Sellers TD. Five-years experience with intravascular lead extraction. U.S. Lead Extraction Database. Pacing Clin Electrophysiol. 1994; 17(11 Pt 2):2016–2020.
5. Wazni O, Epstein LM, Carrillo RG, et al. Lead extraction in the contemporary setting: the LExICon study: an observational retrospective study of consecutive laser lead extractions. J Am Coll Cardiol. 2010; 55:579–586.
6. Neuzil P, Taborsky M, Rezek Z, et al. Pacemaker and ICD lead extraction with electrosurgical dissection sheaths and standard transvenous extraction systems: results of a randomized trial. Europace. 2007; 9:98–104.
7. Wilkoff BL, Love CJ, Byrd CL, et al. Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities, training, indications, and patient management: this document was endorsed by the American Heart Association (AHA). Heart Rhythm. 2009; 6:1085–1104.
8. Yedlicka JW Jr, Carlson JE, Hunter DW, Castañeda-Zúñiga WR, Amplatz K. Nitinol gooseneck snare for removal of foreign bodies: experimental study and clinical evaluation. Radiology. 1991; 178:691–693.
9. Dotter CT, Rösch J, Bilbao MK. Transluminal extraction of catheter and guide fragments from the heart and great vessels; 29 collected cases. Am J Roentgenol Radium Ther Nucl Med. 1971; 111:467–472.
10. Bongiorni MG, Soldati E, Zucchelli G, et al. Transvenous removal of pacing and implantable cardiac defibrillating leads using single sheath mechanical dilatation and multiple venous approaches: high success rate and safety in more than 2000 leads. Eur Heart J. 2008; 29:2886–2893.
11. Zhou X, Jiang H, Ma J, et al. Comparison of standard and modified transvenous techniques for complex pacemaker lead extractions in the context of cardiac implantable electronic device-related infections: a 10-year experience. Europace. 2013; 15:1629–1635.
12. Jarwe M, Klug D, Beregi JP, et al. Single center experience with femoral extraction of permanent endocardial pacing leads. Pacing Clin Electrophysiol. 1999; 22:1202–1209.
13. Klug D, Jarwé M, Messaoudéne SA, et al. Pacemaker lead extraction with the needle's eye snare for countertraction via a femoral approach. Pacing Clin Electrophysiol. 2002; 25:1023–1028.
14. Belott PH. Lead extraction using the femoral vein. Heart Rhythm. 2007; 4:1102–1107.
15. Hauser RG, Kallinen LM, Almquist AK, Gornick CC, Katsiyiannis WT. Early failure of a small-diameter high-voltage implantable cardioverter-defibrillator lead. Heart Rhythm. 2007; 4:892–896.
16. Hauser RG, McGriff D, Retel LK. Riata implantable cardioverter-defibrillator lead failure: analysis of explanted leads with a unique insulation defect. Heart Rhythm. 2012; 9:742–749.
17. Choi K, Kim JH, Kim HJ, Lee SO, Jang EY, Kim JS. A case of riata® dual coil defibrillator lead failure in a patient with ventricular fibrillation. Korean Circ J. 2013; 43:336–339.
18. Maytin M, Love CJ, Fischer A, et al. Multicenter experience with extraction of the Sprint Fidelis implantable cardioverter-defibrillator lead. J Am Coll Cardiol. 2010; 56:646–650.
19. Brunner MP, Cronin EM, Duarte VE, et al. Clinical predictors of adverse patient outcomes in an experience of more than 5000 chronic endovascular pacemaker and defibrillator lead extractions. Heart Rhythm. 2014; 11:799–805.
20. Gomes S, Cranney G, Bennett M, Li A, Giles R. Twenty-year experience of transvenous lead extraction at a single centre. Europace. 2014; 16:1350–1355.
21. Kennergren C, Bjurman C, Wiklund R, Gäbel J. A single-centre experience of over one thousand lead extractions. Europace. 2009; 11:612–617.
22. Epstein AE, Kay GN, Plumb VJ, Dailey SM, Anderson PG. Gross and microscopic pathological changes associated with nonthoracotomy implantable defibrillator leads. Circulation. 1998; 98:1517–1524.
23. Segreti L, Di Cori A, Soldati E, et al. Major predictors of fibrous adherences in transvenous implantable cardioverter-defibrillator lead extraction. Heart Rhythm. 2014; 11:2196–2201.
24. Bordachar P, Defaye P, Peyrouse E, et al. Extraction of old pacemaker or cardioverter-defibrillator leads by laser sheath versus femoral approach. Circ Arrhythm Electrophysiol. 2010; 3:319–323.
25. de Bie MK, Fouad DA, Borleffs CJ, et al. Trans-venous lead removal without the use of extraction sheaths, results of >250 removal procedures. Europace. 2012; 14:112–116.
26. Epstein LM, Love CJ, Wilkoff BL, et al. Superior vena cava defibrillator coils make transvenous lead extraction more challenging and riskier. J Am Coll Cardiol. 2013; 61:987–989.
27. Aoukar PS, Poole JE, Johnson GW, et al. No benefit of a dual coil over a single coil ICD lead: Evidence from the Sudden Cardiac Death in Heart Failure Trial. Heart Rhythm. 2013; 10:970–976.
28. Kutyifa V, Huth Ruwald A-C, Aktas MK, et al. Clinical impact, safety, and efficacy of single- versus dual-coil ICD leads in MADIT-CRT. J Cardiovasc Electrophysiol. 2013; 24:1246–1252.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download