Abstract
Background and Objectives
Subjects and Methods
Results
Figures and Tables
Fig. 1
Study population enrollment. ER: emergency room, WBV: whole blood viscosity, PCI: percutaneous coronary intervention.

Fig. 2
Baseline and one-month follow-up whole blood viscosities (A: SBV, B: DBV). Both the SBV and DBV of group II were significantly higher than those for group I at admission. However, at the one-month follow-up, only the DBV of group II was significantly higher than that for group I. *p<0.05. SBV: systolic blood viscosity, DBV: diastolic blood viscosity.

Fig. 3
Correlation analysis of WBV at admission. Pearson's correlation coefficients were calculated between WBV and various laboratory factors. Among those factors, hematocrit, RBC aggregation, total protein, albumin, ALT, WBC, glucose, rGT, CRP, AST and HbA1c were significantly correlated with WBV (X-axis is R value). *p<0.05, **p<0.01. WBV: whole blood viscosity, RBC: red blood cell, ALT: alanine aminotransferase, WBC: white blood cell, rGT: gamma glutamyl transferase, CRP: C-reactive protein, AST: aspartate aminotransferase, HbA1c: hemoglobin A1c.
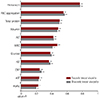
Fig. 4
Correlation analysis of WBV at one-month follow-up. Pearson's correlation coefficients were calculated between WBV and various laboratory factors. Among those factors, hematocrit, albumin, RBC aggregation, ALT, total protein, rGT, CRP, glucose and WBC were significantly correlated with WBV (X-axis is R value). *p<0.05, **p<0.01. WBV: whole blood viscosity, RBC: red blood cell, ALT: alanine aminotransferase, rGT: gamma glutamyl transferase, WBC: white blood cell.
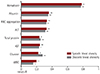
Table 1
Baseline clinical characteristics

Data are expressed as mean±standard deviation or number (%). BMI: body mass index, IHD: ischemic heart disease, CVA: cerebrovascular attack, STEMI: ST-segment elevation myocardial infarction, NSTEMI: non-ST-segment elevation myocardial infarction, UAP: unstable angina pectoris, PCI: percutaneous coronary intervention
Table 2
Comparison of laboratory findings between baseline and one-month follow-up

Data are expressed as mean±standard deviation. WBC: white blood cell, MCV: mean corpuscular volume, MCH: mean corpuscular hemoglobin, ESR: erythrocyte sedimentation rate, rGT: gamma glutamyl transferase, AST: aspartate aminotransferase, TG: triglycerides, LDL-C: low-density lipoprotein-cholesterol, HDL-C: high-density lipoprotein-cholesterol, hs-CRP: high sensitivity C-reactive protein, CK-MB: creatine kinase-MB, RBC: red blood cell, EiMax: elongation index at maximum, SS1/2: shear stress at half maximal deformation, AI: aggregation index, T1/2: aggregation half-time
Table 3
Medications during one-month clinical follow-up





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download