Abstract
Mitral regurgitation (MR) represents the second most frequent valvular heart disease. The appropriate management of organic MR remains unclear in many aspects, especially in several specific clinical scenarios. This review aims to discuss the current guideline recommendations regarding the management of organic MR, while highlighting the controversial aspects encountered in daily clinical practice. The role of imaging is essential in establishing the most appropriate type of surgical treatment (repair or replace), which is based on morphological mitral valve (MV) characteristics (reparability of the valve) and local surgical expertise in valve repair. The potential advantages of 3-dimensional echocardiography in assessing the MV are discussed. Other modern imaging techniques (tissue Doppler and speckle tracking) may provide additional useful information in borderline cases. Exercise echocardiography (evaluating MR severity, pulmonary pressure, or right ventricular function) may have an important role in the management of difficult cases. Finally, the moment when surgery is no longer an option and alternative solutions should be sought is also discussed. Although in everyday clinical practice the timing of surgery is not always straightforward, some newer clinical and echocardiographic indicators can guide this decision and help improve the outcome of these patients.
Mitral regurgitation (MR) represents the second most frequent valvular heart disease.1)2) The appropriate management of organic MR remains controversial in many aspects, especially in several specific clinical scenarios. The prognosis of patients with severe MR is poor without surgery,3)4) while in patients with successful mitral valve (MV) repair, there is no difference from their normal expected survival.5) The ideal treatment for MR is MV repair, but in everyday clinical practice some important questions need to be answered:
This review aims to discuss the current guideline recommendations regarding the management of organic MR, while highlighting the controversial aspects encountered in daily clinical practice and the role of imaging in borderline cases.
Guidelines for the management of patients with organic and functional MR have been recently published both by the American Heart Association (AHA)/American College of Cardiology (ACC) in 20146) and by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)7) in 2012 (Table 1). The decision for surgery in a patient with severe MR is a complex process, requiring one to consider many variables including the severity of MR, the patient's symptoms, the impact on ventricular and atrial dimensions, shape and function of the valve and its surrounding anatomy, pulmonary pressures, the feasibility of a successful repair, comorbidities, and operative risk. Both guidelines recommend MV repair as the preferred surgical treatment. Surgery is indicated for patients with severe symptomatic MR in the absence of severe left ventricular (LV) dysfunction {LV ejection fraction (EF) >30%}, or for patients with severe asymptomatic MR and LV geometrical changes (LV end-systolic diameter >40 mm in the AHA/ACC guidelines; or >45 mm in patients with prolapse and >40 mm in patients with flail leaflet in the ESC guidelines), LV systolic dysfunction (LVEF <60%), new onset atrial fibrillation, or pulmonary hypertension {systolic pulmonary artery pressure (SPAP) >50 mm Hg}.6)7) Discrepancies between the two guidelines arise regarding the patients with severe symptomatic MR and LVEF less than 30%, or the patients with severe asymptomatic MR, LVEF more than 60%, or sinus rhythm and pulmonary pressure less than 50 mm Hg. In these two situations, the likelihood of a durable MV repair plays an essential role.
The contemporary data from reference centers for MV repair have demonstrated an operative mortality for isolated elective MV repair less than 1%,8) with a repair rate close to 100%.9)10) Therefore, the new trend in modern MV treatment is surgical repair for all relevant patients. However, most of the centers have reported a lower repair rate, which seems to reflect each center's volume of patients and the individual surgeon's experience.11)12) In clinical practice, the likelihood of a durable MV repair is evaluated by taking into account the morphological MV appearance on echocardiography together with the surgeon's and center's experience (Table 2). The heart team, formed by a cardiologist (proficient in the advanced echocardiographic evaluation of the MV), an experienced surgeon (who performs more than 50 MV repair interventions per year), and an experienced intensive care unit is essential for a successful repair.
There are echocardiographic criteria demonstrated as being indicators for low likelihood of MV repair: the presence of a large central regurgitant jet, severe annular dilatation (i.e., diastolic anteroposterior annulus diameter more than 50 mm), and the involvement of ≥3 scallops, especially when the anterior leaflet is affected and there are extensive valve calcifications.13)
To conclude, MV lesions in organic MR can be classified as: simple lesions (e.g., isolated posterior leaflet prolapse or flail-leaflet perforation); complex lesions (e.g., anterior prolapse or flail, complex posterior prolapse, bileaflet prolapse, commissural prolapse, or combined lesions); or very complex lesions (e.g., extensive prolapse, prolapse with hypoplasia of the opposite leaflet, post endocarditic extensive destruction, or rheumatic disease) (Fig. 1) (Supplementary Videos 1, 2, and 3 in the online-only Data Supplement).
The probability of successful repair is high in simple lesions, while success depends on the experience of the surgical team in complex lesions, and is very low in very complex lesions.15)
The development of three-dimensional (3D) echocardiography, especially transesophageal 3D echocardiography, offers a great advantage over two-dimensional (2D) echocardiography with the possibility to visualize 'en face' the entire MV, similar to the intraoperative surgical view,16) permitting a more accurate assessment of the extent and location of the disease (Fig. 2) (Supplementary Videos 4 and 5 in the online-only Data Supplement).17) Moreover, the newly developed software based on 3D echocardiography transforms the MV in a mathematical model (Fig. 3), providing specific measurements essential for the surgeon (annulus dimensions, non-planar angle, leaflets area, or tenting height and volume). The quantification of MR severity by 3D echocardiography (3D vena contracta or regurgitant volume calculation) is feasible and superior to 2D methods, compared to gold standard MRI.18)
The timing of an intervention in severe asymptomatic organic MR is a complex and hotly debated issue. A randomized trial comparing the "watchful waiting" strategy with the "early intervention" strategy15) has not been performed yet. The results of contemporary observational studies are contradictory. Some results support the idea that patients with asymptomatic severe MR can be safely followed up until symptoms develop or currently recommended cut-off values for LV size, LV function, or pulmonary hypertension are reached.19) Conversely, other studies show that early surgery is associated with improved long-term survival and lower rates of hospitalization for congestive heart failure.20)21)22)23) The recently published AHA/ACC guidelines on valvular heart disease recommend intervention for all patients with chronic severe primary MR with preserved LV function (EF >60% and LV end-systolic diameter <40 mm) in whom the likelihood of a successful and durable repair without residual MR is >95% with an expected mortality rate of <1% when performed at a Heart Valve Center of Excellence.6)
In actual practice, the Heart Valve Center of Excellence may not be available and there is uncertainty regarding patients who do not meet the current guidelines' cut-off values for intervention. What are the additional tools available to further stratify their risk and to decide the best time for surgery?
Brain natriuretic peptide (BNP) is released by cardiac myocytes in response to increased myocardial wall stress and consecutive cell stretching.24) Therefore the elevation of this peptide indicates a volume overload and possible subclinical myocardial function impairment.24)25)26) Several studies have demonstrated that BNP levels are associated with outcome in patients with severe asymptomatic MR (Table 3).
The evaluation of LV size and function is a mandatory step for the proper management of a patient with severe asymptomatic MR. Current guidelines recommend surgery for patients with LV systolic dysfunction (defined as LVEF <60%), or LV dilatation.6)7) It is important to identify among those patients with severe MR, those at risk of postoperative LV dysfunction because LV systolic dysfunction after surgery predicts poor short- and long-term outcomes. Thus, the early recognition of LV contractile dysfunction followed by appropriate surgical correction of MR may avoid the development of irreversible postoperative LV damage.
Therefore, newer echocardiographic methods (e.g., global longitudinal, circumferential and radial strain, strain rate, LV torsion, and untwist) used to assess subclinical LV systolic dysfunction29)30) may be helpful in determining the timing for valvular intervention and to avoid the development of overt postoperative LV dysfunction.
The global longitudinal strain (GLS) measures the longitudinal deformation of the LV and represents the percentage change in dimension in systole compared to diastole. Mascle et al.31) demonstrated that, in patients with severe asymptomatic MR and normal LVEF, GLS measured by speckle tracking echocardiography is an independent predictor for LV systolic dysfunction after surgery: a reduced deformation (GLS below -18%) predicts with a sensitivity of 53% and a specificity of 76% a post MV repair LVEF lower than 50% (Fig. 4) (Supplementary Video 6 in the online-only Data Supplement). Likewise, Witkowski et al.32) reported that a GLS below -19.9% could predict LV dysfunction with a sensitivity and specificity of 90% and 79%, respectively.
Conversely, Pandis et al.33) have recently demonstrated that a higher preoperative GLS in a patient with severe asymptomatic MR represents a disproportionately preload-related compensation in the longitudinal direction and this may indicate a risk for a substantial reduction in LVEF immediately following MV repair. An increased deformation (GLS above -20.5%) predicts a drop in LVEF >10% with a sensitivity of 66.7% and a specificity of 73%. A GLS below -17.9% predicts a LVEF <50% with a sensitivity of 80.7% and a specificity of 100%. Moreover, preoperative measurements of LV circumferential and radial mechanics did not predict LV dysfunction after MV repair.
To conclude, a higher, as well as a lower, value for GLS may predict LV systolic dysfunction after MV surgery. Further studies are needed to standardize and determine reliable cut-off values before GLS can be used in the management of patients with MR in clinical practice.
The LV twist and untwist represent the complex wringing motion of the LV during the cardiac cycle, which increases the efficiency of cardiac performance and has an important role in both systolic ejection and diastolic filling.26) LV twist measures the apex-to-base difference in rotation (expressed in degrees), while LV torsion represents the base-to-apex gradient in the rotational angle along the long axis (expressed in degrees per centimeter). These parameters can be measured by cardiac magnetic resonance with tissue tagging or by speckle tracking echocardiography.26)
Using speckle tracking echocardiography, Moustafa et al.34) have shown that, in patients with moderate organic MR, the LV rotation profile is high, indicating a hyperdynamic LV function. In comparison, in severe MR, the LV rotation profile is the lowest, suggesting incipient LV dysfunction. Therefore, LV torsion may represent a useful tool for unmasking incipient LV systolic dysfunction. Significant delays in the onset and peak of LV untwisting were reported by Borg et al.35) in patients with chronic moderate-severe MR due to MV prolapse and correlations between disease severity and torsional parameters suggest a potential role of these measurements in identifying early signs of ventricular dysfunction. If the issues related to the standardization of their use are solved, these parameters may become promising indicators for the timing of surgery in severe asymptomatic MR.
The response of the left atrium to volume overload in severe MR has been extensively studied. Left atrium dimensions (left atrium diameter >55 mm or left atrium index >60 mL/m2)36)37) predict long term mortality in patients with organic MR regardless of MR severity, symptoms, LV size and function, atrial fibrillation, or pulmonary hypertension. Therefore, the current ESC guidelines for the management of valvular heart disease recommend surgical treatment for asymptomatic patients with severe MR, a high likelihood of MV repair, and left atrium dilation (class IIb),7) while the AHA/ACC guidelines have no specific indications regarding left atrium size.6)
Recently published data by Ring et al.38) underscore the possible role of assessing left atrium function in managing patients with severe MR. The left atrium adapts to severe MR initially by dilatation with preserved function. The decrease of normal atrial function (estimated by the left atrium emptying fraction calculated using the Simpson's method, contractile, and reservoir function using 2D strain) is highly predictive for the need of surgery in organic MR (Fig. 5). A left atrium emptying fraction <50% has a sensitivity of 91% and a specificity of 92% for predicting surgical indication in organic MR. The assessment of left atrium function in everyday clinical practice may become a useful tool in determining the optimal timing for surgery in MR.39) Left atrium function estimated by speckle tracking-derived peak atrial longitudinal strain has been shown to correlate strongly with the extent of left atrium fibrosis demonstrated through histology using tissue samples in a group of patients treated surgically for severe MR.40)
The ESC7) as well as the AHA/ACC6) guidelines regarding valvular heart disease recommend surgical treatment of severe MR for patients with resting pulmonary hypertension (SPAP >50 mm Hg) (class IIa). Pulmonary hypertension is a poor prognostic factor, doubling the risk of death and heart failure after diagnosis and decreasing early and late survival after MV operations.41)42) MR correction is beneficial in patients with or without pulmonary hypertension but the most favorable postsurgical outcome is in patients with normal pulmonary pressure. Therefore, it is desirable to perform surgery before pulmonary hypertension develops. Residual pulmonary hypertension after MV surgery is a poor outcome indicator43) and patient-prosthesis mismatch may be a risk factor for persistent pulmonary hypertension after surgery.44) MV repair offers favorable hemodynamics, avoiding patient-prosthesis mismatch, and the rate of postoperative pulmonary hypertension is consequently lower.45)
There is no data regarding a level of pulmonary hypertension beyond which MV surgery would be contraindicated. However, pulmonary hypertension is one of the parameters included in the Euro Score calculation to predict the risk of surgical intervention.
Magne et al.46)47) have demonstrated that exercise pulmonary hypertension predicts the occurrence of symptoms in severe asymptomatic MR. The ESC guidelines7) for the management of valvular heart disease recommend surgical correction of MR in patients with a high likelihood of durable repair, low surgical risk, and exercise pulmonary hypertension >60 mm Hg, while the ACC/AHA guidelines6) have no specific recommendations regarding this category.
Right ventricular (RV) size can be estimated by RV end-diastolic and end-systolic area, and RV resting function can be estimated by fractional area change, RV strain and TAPSE are predictors for surgery in asymptomatic patients with severe MR.48)49) Moreover, Kusunose et al.50) has shown that, in patients with severe MR without classical criteria for surgery (i.e., symptoms, LV dysfunction, atrial fibrillation, pulmonary hypertension), exercise RV dysfunction (estimated by TAPSE <19 mm on exercise) provides additional value to exercise-induced pulmonary hypertension in the prediction of time until surgery is indicated.
There are no clear contraindications for MV surgery in patients with severe symptomatic MR. According to the ESC guidelines, in patients with severe systolic dysfunction (LVEF <30%), MV repair is indicated when the likelihood of successful repair is high (class IIa), whereas when the likelihood of repair is low, the recommendation for surgery is weaker (class IIb). The ACC/AHA guidelines give a class IIb recommendation for MV surgery in patients with severe MR and severe systolic dysfunction, regardless of the probability of MV repair.
In patients with severe symptomatic MR and high surgical risk or severe comorbidities, but otherwise reasonable life expectancy, the surgical risk may be prohibitive. Both guidelines suggest the possibility of interventional treatment. The high surgical risk is generally defined by a logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE) mortality >15%, or the presence of specific surgical risk factors not covered by the EuroSCORE (i.e., frailty, immunosuppressive therapy, porcelain aorta, or extensive mediastinal radiation).51) Percutaneous MV replacement can be performed using the MitraClip system (Evalve, Menlo Park, CA, USA), which is currently the only percutaneous device available for clinical use.52) The procedure involves the implantation of one or more clips at the site of regurgitation, similar to the surgical edge-to-edge repair described by Alfieri.53) It requires a triaxial delivery system introduced through the femoral vein and positioned by trans-septal puncture into the left atrium54) under fluoroscopic and transoesophageal guidance.55) In organic MR, the MV morphology suitable for Mitra-Clip, as defined by the Everest criteria56)57) should meet the following conditions: sufficient leaflet tissue for mechanical coaptation, resting MV effective orifice area >4 cm2, coaptation length >2 mm, flail gap, in case of MV flail, <10 mm, and flail width <15 mm. The rheumatic etiology of MR and patients with calcified leaflets were excluded from clinical trials.
The last randomized, controlled trial (Endovascular Valve Edge-to-Edge Repair Study II) comparing percutaneous treatment of MR with conventional surgery showed that percutaneous repair was less effective than surgery in reducing MR before hospital discharge. At 12 and 24 months, the rates of reduction in MR were similar, and percutaneous treatment was associated with increased safety, improved LV dimensions, better New York Heart Association class, and an improved quality of life.57) Overall, at the 4 year follow-up, MV reoperation for residual MR was more frequent in the percutaneous treatment group compared to the surgical group, but the prevalence of severe MR and mortality were not significantly different. However, after the first year of follow-up, there were only a few cases requiring surgery after either percutaneous or surgical treatment.58)
The treatment of patients with organic MR still poses many challenges and controversies, as many findings and recommendations have not yet been supported by solid evidence. MV repair is the treatment of choice in severe degenerative MR; however, it is not possible in all cases. In everyday clinical practice, the timing of the surgery is still a matter of debate, based on factors such as the repairability of the valve, the size and function of the LV and, importantly, local surgical expertise in valve repair. Some newer clinical and echocardiographic indicators can guide this decision and help improve the outcome of these patients.
Figures and Tables
Fig. 1
Mitral valve lesions in severe organic mitral regurgitation, assessed by three-dimensional transoesophageal echocardiography. A: severe mitral regurgitation determined by a simple lesion with a high probability of successful mitral valve repair. 3D transoesophageal surgical view of the mitral valve showing isolated P2 scallop prolapse (asterisk) (Supplementary Video 1 in the online-only Data Supplement). B: severe mitral regurgitation determined by complex lesions with a possibly successful mitral valve repair by an experienced surgeon. 3D transoesophageal surgical view of the mitral valve showing P3 scallop prolapse and flail (asterisk) involving the posterior commissure (Supplementary Video 2 in the online-only Data Supplement). C: severe mitral regurgitation determined by a very complex lesion with an unlikely chance of successful mitral valve repair. 3D transoesophageal surgical view in a patient with Barlow disease and P2 flail (asterisk) (Supplementary Video 3 in the online-only Data Supplement). 3D: three-dimensional.

Fig. 2
Mitral valve assessment in a patient with severe mitral regurgitation. A: 2D transoesophageal four chamber view of posterior mitral valve prolapse and flail due to chordal rupture (arrow) (Supplementary Video 4 in the online-only Data Supplement). B: 3D transoesophageal view of the mitral valve seen from the left atrium showing isolated P2 prolapse and flail (asterisk) (Supplementary Video 5 in the online-only Data Supplement). C: 3D mitral valve reconstruction demonstrating P2 prolapse (color coded in red). D: intraoperative findings confirming the echo results: P2 scallop chordal rupture (asterisk). 2D: two-dimensional, 3D: three-dimensional.
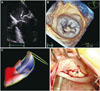
Fig. 3
Mitral valve reconstruction in a normal subject (A) and in a patient with severe mitral regurgitation due to P2 scallop flail and prolapse and P3 scallop prolapse (B). The parts of the mitral valve which are below the mitral annulus plane (i.e., on the ventricular side) are color-coded in blue, while the parts which are above annulus are coded in red. Of note, the shape of the mitral annulus changes in MR, becoming circular (B), compared to the oval shape of the normal mitral annulus (A). MR: metral regurgitation.

Fig. 4
Echocardiographic images from a patient with severe asymptomatic mitral regurgitation. There is a preserved left ventricular (LV) ejection fraction calculated by Simpson's method (61%) (A), but reduced global longitudinal strain (-14.3%) (B), suggesting subclinical LV systolic dysfunction (Supplementary Video 6 in the online-only Data Supplement). LVEF: left ventricular ejection fraction, SV: stroke volume, LVESV: left ventricular end systolic volume, LVEDV: left ventricular end diastolic volume.
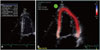
Fig. 5
Left atrium (LA) function evaluation in a patient with severe asymptomatic mitral regurgitation (MR). The LA ejection fraction calculated by LA maximum volume (130 mL in A)-LA minimum volume (80 mL in B) divided by LA maximum volume is decreased to 38%. The LA strain values (i.e., reservoir, conduit, and contractile function) calculated by speckle tracking imaging (C) in the same patient with severe asymptomatic MR are decreased.

Table 1
ESC/EACTS and AHA/ACC guidelines for valve disease management

| 2012 ESC/EACTS guidelines7) | 2014 AHA/ACC guidelines6) |
|---|---|
| Intervention in symptomatic patients | |
| Surgery is indicated in symptomatic patients with LVEF >30% and LVESD | MV surgery is recommended for symptomatic patients with chronic severe primary MR (stage D) and LVEF >30% (IB) |
| Surgery should be considered in patients with severe LV dysfunction (LVEF<30% and/ or LVESD >55 mm) refractory to medical therapy with a high likelihood of durable repair and low comorbidity (IIaC) | MV surgery may be considered in symptomatic patients with chronic severe primary MR and LVEF<30% (stage D) (IIbC) |
| Surgery may be considered in patients with severe LV dysfunction (LVEF<30% and/or LVESD >55 mm) refractory to medical therapy with a low likelihood of durable repair and low comorbidity (IIbC) | Transcatheter MV repair may be considered for severely symptomatic patients (NYHA class III/IV) with chronic severe primary MR (stage D) who have a reasonable life expectancy but a prohibitive surgical risk because of severe comorbidities (IIbB) |
| Intervention in asymptomatic patients | |
| Surgery is indicated in asymptomatic patients with LV dysfunction (LVESD ≥45 mm and/or LVEF ≤60%) (IC) | MV surgery is recommended for asymptomatic patients with chronic severe primary MR and LV dysfunction (LVEF 30-60% and/or LVESD ≥40 mm, stage C2) (IB) |
| Surgery should be considered in asymptomatic patients with preserved LV function, high likelihood of durable repair, low surgical risk, and flail leaflet and LVESD ≥40 mm (IIaC) | MV repair is reasonable for asymptomatic patients with chronic severe nonrheumatic primary MR (stage C1) and preserved LV function in whom there is a high likelihood of a successful and durable repair with |
| Surgery should be considered in asymptomatic patients with preserved LV function and | 1) new onset of AF, or |
| · new onset of AF or | 2) resting pulmonary hypertension (PA systolic arterial pressure >50 mm Hg) (IIaB) |
| · pulmonary hypertension (systolic pulmonary pressure at rest >50 mm Hg) (IIaC) | MV repair is reasonable in asymptomatic patients with chronic severe primary MR (stage C1) with preserved LV function (LVEF >60% and LVESD 95% with an expected mortality rate of <1% when performed at a Heart Valve Center of Excellence (IIaB) |
| Surgery may be considered in asymptomatic patients with preserved LV function, high likelihood of durable repair, low surgical risk, and: | |
| · left atrial dilatation (volume index ≥60 mL/m2 BSA) and sinus rhythm, or | |
| · pulmonary hypertension on exercise (SPAP ≥60 mm Hg at exercise) (IIbC) | |
| Intervention type: repair vs. replace | |
| Mitral valve repair should be the preferred technique when it is expected to be durable (IC) | MV repair is recommended in preference to MVR when surgical treatment is indicated for patients with chronic severe primary MR limited to the posterior leaflet (IB) |
| MV repair is recommended in preference to MVR when surgical treatment is indicated for patients with chronic severe primary MR involving the anterior leaflet or both leaflets when a successful and durable repair can be accomplished (IB) | |
| MV repair may be considered in patients with rheumatic mitral valve disease when surgical treatment is indicated, if a durable and successful repair is likely or if the reliability of long-term anticoagulation management is questionable (IIbB) | |
| MVR should not be performed for treatment of isolated severe primary MR limited to less than one half of the posterior leaflet unless MV repair has been attempted and was unsuccessful (IIIB) |
ESC/EACTS: European Society of Cardiology/European Association for Cardio-Thoracic Surgery, AHA/ACC: American Heart Association/American College of Cardiology, LVEF: left ventricle ejection fraction, LVESD: left ventricle end-systolic diameter, MV: mitral valve, MR: mitral regurgitation, LV: left ventricle, NYHA: New York Heart Association, AF: atrial fibrillation, PA: pulmonary artery, BSA: body surface area, SPAP: systolic pulmonary artery pressure, MVR: mitral valve replacement
Table 2

Table 3
Role of brain natriuretic peptide levels in decision making for patients with organic mitral regurgitation

| Study | Year | Pts | Inclusion criteria | End point | Cut-off value |
|---|---|---|---|---|---|
| Pizarro et al.24) | 2009 | 269 | Asymptomatic severe MR | HF, LV dysfunction, death | 105 pg/mL |
| EF >60% | |||||
| Detaint et al.25) | 2005 | 126 | Organic MR (symptomatic/asymptomatic) | HF, death | 31 pg/mL |
| Klaar et al.26) | 2011 | 87 | Asymptomatic severe MR | HF | 145 pg/mL |
| EF >60% | LV dysfunction | ||||
| LV end-systolic diameter index <26 mm/m2, SPAP <50 mm Hg, no atrial fibrillation | |||||
| Magne et al.27) | 2012 | 135 | Asymptomatic moderate/severe MR | Cardiac event free survival | 40 pg/mL |
| Magne et al.28) | 2012 | 113 | Asymptomatic moderate/severe MR | Death, HF, mitral valve surgery due to symptoms, LV dilatation, LV dysfunction | Increasing BNP level at exercise |
Acknowledgments
Popescu BA has received research support and lecture honoraria from General Electric Healthcare.
This work was supported by a grant of the Romanian Ministry of National Education, CNCS-UEFISCDI, project number PN-II-ID-PCE-2012-4-0560 (contract 21/2013), and by the Sectorial Operational Programme Human Resources Development (SOP HRD), financed by the European Social Fund and by the Romanian Government under the contract number POSDRU/159/1.5/S/132395.
References
1. Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003; 24:1231–1243.
2. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006; 368:1005–1011.
3. Delahaye JP, Gare JP, Viguier E, Delahaye F, De Gevigney G, Milon H. Natural history of severe mitral regurgitation. Eur Heart J. 1991; 12:Suppl B. 5–9.
4. Tribouilloy CM, Enriquez-Sarano M, Schaff HV, et al. Impact of preoperative symptoms on survival after surgical correction of organic mitral regurgitation: rationale for optimizing surgical indications. Circulation. 1999; 99:400–405.
5. Enriquez-Sarano M, Schaff HV, Orszulak TA, Tajik AJ, Bailey KR, Frye RL. Valve repair improves the outcome of surgery for mitral regurgitation. A multivariate analysis. Circulation. 1995; 91:1022–1028.
6. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014; 63:2438–2488.
7. Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC). European Association for Cardio-Thoracic Surgery (EACTS). Vahanian A, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. 2012; 33:2451–2496.
8. Gammie JS, Sheng S, Griffith BP, et al. Trends in mitral valve surgery in the United States: results from the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann Thorac Surg. 2009; 87:1431–1437. discussion 1437-9.
9. Castillo JG, Anyanwu AC, Fuster V, Adams DH. A near 100% repair rate for mitral valve prolapse is achievable in a reference center: implications for future guidelines. J Thorac Cardiovasc Surg. 2012; 144:308–312.
10. Castillo JG, Anyanwu AC, El-Eshmawi A, Adams DH. All anterior and bileaflet mitral valve prolapses are repairable in the modern era of reconstructive surgery. Eur J Cardiothorac Surg. 2014; 45:139–145. discussion 145.
11. Gammie JS, O'Brien SM, Griffith BP, Ferguson TB, Peterson ED. Influence of hospital procedural volume on care process and mortality for patients undergoing elective surgery for mitral regurgitation. Circulation. 2007; 115:881–887.
12. Bolling SF, Li S, O'Brien SM, Brennan JM, Prager RL, Gammie JS. Predictors of mitral valve repair: clinical and surgeon factors. Ann Thorac Surg. 2010; 90:1904–1911. discussion 1912.
13. Omran AS, Woo A, David TE, Feindel CM, Rakowski H, Siu SC. Intraoperative transesophageal echocardiography accurately predicts mitral valve anatomy and suitability for repair. J Am Soc Echocardiogr. 2002; 15:950–957.
14. Lancellotti P, Tribouilloy C, Hagendorff A, et al. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2013; 14:611–644.
15. De Bonis M, Bolling SF. Mitral valve surgery: wait and see vs. early operation. Eur Heart J. 2013; 34:13–19a.
16. Vegas A, Meineri M. Core review: three-dimensional transesophageal echocardiography is a major advance for intraoperative clinical management of patients undergoing cardiac surgery: a core review. Anesth Analg. 2010; 110:1548–1573.
17. Pepi M, Tamborini G, Maltagliati A, et al. Head-to-head comparison of two- and three-dimensional transthoracic and transesophageal echocardiography in the localization of mitral valve prolapse. J Am Coll Cardiol. 2006; 48:2524–2530.
18. Shanks M, Siebelink HM, Delgado V, et al. Quantitative assessment of mitral regurgitation: comparison between three-dimensional transesophageal echocardiography and magnetic resonance imaging. Circ Cardiovasc Imaging. 2010; 3:694–700.
19. Rosenhek R, Rader F, Klaar U, et al. Outcome of watchful waiting in asymptomatic severe mitral regurgitation. Circulation. 2006; 113:2238–2244.
20. Kang DH, Kim JH, Rim JH, et al. Comparison of early surgery versus conventional treatment in asymptomatic severe mitral regurgitation. Circulation. 2009; 119:797–804.
21. Suri RM, Vanoverschelde JL, Grigioni F, et al. Association between early surgical intervention vs watchful waiting and outcomes for mitral regurgitation due to flail mitral valve leaflets. JAMA. 2013; 310:609–616.
22. Tietge WJ, de Heer LM, van Hessen MW, et al. Early mitral valve repair versus watchful waiting in patients with severe asymptomatic organic mitral regurgitation; rationale and design of the Dutch AMR trial, a multicenter, randomised trial. Neth Heart J. 2012; 20:94–101.
23. Kang DH, Park SJ, Sun BJ, et al. Early surgery versus conventional treatment for asymptomatic severe mitral regurgitation: a propensity analysis. J Am Coll Cardiol. 2014; 63:2398–2407.
24. Pizarro R, Bazzino OO, Oberti PF, et al. Prospective validation of the prognostic usefulness of brain natriuretic peptide in asymptomatic patients with chronic severe mitral regurgitation. J Am Coll Cardiol. 2009; 54:1099–1106.
25. Detaint D, Messika-Zeitoun D, Avierinos JF, et al. B-type natriuretic peptide in organic mitral regurgitation: determinants and impact on outcome. Circulation. 2005; 111:2391–2397.
26. Klaar U, Gabriel H, Bergler-Klein J, et al. Prognostic value of serial B-type natriuretic peptide measurement in asymptomatic organic mitral regurgitation. Eur J Heart Fail. 2011; 13:163–169.
27. Magne J, Mahjoub H, Pierard LA, et al. Prognostic importance of brain natriuretic peptide and left ventricular longitudinal function in asymptomatic degenerative mitral regurgitation. Heart. 2012; 98:584–591.
28. Magne J, Mahjoub H, Pibarot P, Pirlet C, Pierard LA, Lancellotti P. Prognostic importance of exercise brain natriuretic peptide in asymptomatic degenerative mitral regurgitation. Eur J Heart Fail. 2012; 14:1293–1302.
29. Reisner SA, Lysyansky P, Agmon Y, Mutlak D, Lessick J, Friedman Z. Global longitudinal strain: a novel index of left ventricular systolic function. J Am Soc Echocardiogr. 2004; 17:630–633.
30. Beladan CC, Călin A, Roşca M, Ginghină C, Popescu BA. Left ventricular twist dynamics: principles and applications. Heart. 2014; 100:731–740.
31. Mascle S, Schnell F, Thebault C, et al. Predictive value of global longitudinal strain in a surgical population of organic mitral regurgitation. J Am Soc Echocardiogr. 2012; 25:766–772.
32. Witkowski TG, Thomas JD, Debonnaire PJ, et al. Global longitudinal strain predicts left ventricular dysfunction after mitral valve repair. Eur Heart J Cardiovasc Imaging. 2013; 14:69–76.
33. Pandis D, Sengupta PP, Castillo JG, et al. Assessment of longitudinal myocardial mechanics in patients with degenerative mitral valve regurgitation predicts postoperative worsening of left ventricular systolic function. J Am Soc Echocardiogr. 2014; 27:627–638.
34. Moustafa SE, Kansal M, Alharthi M, Deng Y, Chandrasekaran K, Mookadam F. Prediction of incipient left ventricular dysfunction in patients with chronic primary mitral regurgitation: a velocity vector imaging study. Eur J Echocardiogr. 2011; 12:291–298.
35. Borg AN, Harrison JL, Argyle RA, Ray SG. Left ventricular torsion in primary chronic mitral regurgitation. Heart. 2008; 94:597–603.
36. Rusinaru D, Tribouilloy C, Grigioni F, et al. Left atrial size is a potent predictor of mortality in mitral regurgitation due to flail leaflets: results from a large international multicenter study. Circ Cardiovasc Imaging. 2011; 4:473–481.
37. Le Tourneau T, Messika-Zeitoun D, Russo A, et al. Impact of left atrial volume on clinical outcome in organic mitral regurgitation. J Am Coll Cardiol. 2010; 56:570–578.
38. Ring L, Rana BS, Wells FC, Kydd AC, Dutka DP. Atrial function as a guide to timing of intervention in mitral valve prolapse with mitral regurgitation. JACC Cardiovasc Imaging. 2014; 7:225–232.
39. Bonow RO. Left atrial function in mitral regurgitation: guilt by association. JACC Cardiovasc Imaging. 2014; 7:233–235.
40. Cameli M, Lisi M, Righini FM, et al. Usefulness of atrial deformation analysis to predict left atrial fibrosis and endocardial thickness in patients undergoing mitral valve operations for severe mitral regurgitation secondary to mitral valve prolapse. Am J Cardiol. 2013; 111:595–601.
41. Ghoreishi M, Evans CF, DeFilippi CR, et al. Pulmonary hypertension adversely affects short- and long-term survival after mitral valve operation for mitral regurgitation: implications for timing of surgery. J Thorac Cardiovasc Surg. 2011; 142:1439–1452.
42. Barbieri A, Bursi F, Grigioni F, et al. Prognostic and therapeutic implications of pulmonary hypertension complicating degenerative mitral regurgitation due to flail leaflet: a multicenter long-term international study. Eur Heart J. 2011; 32:751–759.
43. Crawford FA Jr. Residual pulmonary artery hypertension after mitral valve replacement: size matters! J Am Coll Cardiol. 2005; 45:1041–1042.
44. Li M, Dumesnil JG, Mathieu P, Pibarot P. Impact of valve prosthesis-patient mismatch on pulmonary arterial pressure after mitral valve replacement. J Am Coll Cardiol. 2005; 45:1034–1040.
45. Walls MC, Cimino N, Bolling SF, Bach DS. Persistent pulmonary hypertension after mitral valve surgery: does surgical procedure affect outcome? J Heart Valve Dis. 2008; 17:1–9. discussion 9.
46. Magne J, Lancellotti P, O'Connor K, Van de Heyning CM, Szymanski C, Piérard LA. Prediction of exercise pulmonary hypertension in asymptomatic degenerative mitral regurgitation. J Am Soc Echocardiogr. 2011; 24:1004–1012.
47. Magne J, Lancellotti P, Piérard LA. Exercise pulmonary hypertension in asymptomatic degenerative mitral regurgitation. Circulation. 2010; 122:33–41.
48. Haddad F, Doyle R, Murphy DJ, Hunt SA. Right ventricular function in cardiovascular disease, part II: pathophysiology, clinical importance, and management of right ventricular failure. Circulation. 2008; 117:1717–1731.
49. Shiran A, Sagie A. Tricuspid regurgitation in mitral valve disease incidence, prognostic implications, mechanism, and management. J Am Coll Cardiol. 2009; 53:401–408.
50. Kusunose K, Popović ZB, Motoki H, Marwick TH. Prognostic significance of exercise-induced right ventricular dysfunction in asymptomatic degenerative mitral regurgitation. Circ Cardiovasc Imaging. 2013; 6:167–176.
51. Sürder D, Pedrazzini G, Gaemperli O, et al. Predictors for efficacy of percutaneous mitral valve repair using the MitraClip system: the results of the MitraSwiss registry. Heart. 2013; 99:1034–1040.
52. Messika-Zeitoun D. Percutaneous mitral valve repair using the Mitra-Clip system: time to move forward. Heart. 2013; 99:975–976.
53. Alfieri O, Maisano F, De Bonis M, et al. The double-orifice technique in mitral valve repair: a simple solution for complex problems. J Thorac Cardiovasc Surg. 2001; 122:674–681.
54. De Bonis M, Maisano F, La Canna G, Alfieri O. Treatment and management of mitral regurgitation. Nat Rev Cardiol. 2011; 9:133–146.
55. Guarracino F, Baldassarri R, Ferro B, et al. Transesophageal echocardiography during MitraClip® procedure. Anesth Analg. 2014; 118:1188–1196.
56. Feldman T, Wasserman HS, Herrmann HC, et al. Percutaneous mitral valve repair using the edge-to-edge technique: six-month results of the EVEREST Phase I Clinical Trial. J Am Coll Cardiol. 2005; 46:2134–2140.
57. Feldman T, Foster E, Glower DD, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011; 364:1395–1406.
58. Mauri L, Foster E, Glower DD, et al. 4-year results of a randomized controlled trial of percutaneous repair versus surgery for mitral regurgitation. J Am Coll Cardiol. 2013; 62:317–328.
Supplementary Material
The online-only Data Supplement is available with this article at http://dx.doi.org/10.4070/kcj.2015.45.2.96.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download