Abstract
Isolated left ventricular noncompaction (LVNC) is a rare cardiomyopathy with morphologic characteristics of two distinct myocardial layers i.e., thin compacted epicardial and thick noncompacted endocardial layers. The noncompacted myocardium consists of prominent ventricular trabeculae and deep intertrabecular recesses. It can lead to arrhythmias, heart failure or systemic embolisms. Electrocardiographic patterns of patients with LVNC are various and non-specific; however, the most common findings are intraventricular conduction delay, left ventricular hypertrophy, and repolarization abnormalities. We reported the first case, to the best of our knowledge, of a 29-year-old man who had recent cerebral infarction and incidental LVNC with spontaneous left atrial standstill.
Left ventricular noncompaction (LVNC) is a rare primary congenital cardiomyopathy arising from intrauterine arrest of myocardial compaction. It results in persistent prominent ventricular trabeculations and deep intertrabecular recesses and is characterized morphologically by 2 layers i.e., thin compacted epicardial layer and thickened noncompacted endocardial layer.1) The prevalence of LVNC is approximately 0.05%-0.24% in the general population according to echocardiography, the diagnostic tool of LVNC.2) The etiology of LVNC is unidentified; however, possible linkage between the disease and various genetic background is recently suspected.3) Diverse clinical manifestations previously reported range from no symptom to the development of heart failure, arrhythmias, systemic embolic events or sudden cardiac deaths.3) Interestingly, several abnormal electrocardiograms (ECGs) were reported in LVNC patients but none had the feature of left atrial (LA) standstill imitating atrioventricular (AV) nodal block. We described a 29-year-old man who presented with a symptom of cerebrovascular accident (CVA) and concomitant asymptomatic LVNC and LA standstill.
A 29-year-old man with the history of stroke was transferred from a local medical center to determine whether he should receive pacemaker insertion due to abnormal ECG findings. At first, he was admitted to a local clinic with dysarthria a month prior to the transfer, and was diagnosed as left middle cerebral artery territory infarction. His brain magnetic resonance imaging (MRI) demonstrated multiple cortical infarcts implying embolic origin (Fig. 1). Magnetic resonance angiography findings showed no evidence of pathologic stenosis or atheroma of the neck and head arteries. Asymptomatic bradycardia was shown on the local ECG with 2:1 AV block reported on local Holter monitoring (SEER light, GE Medical, USA). There was neither specific family history nor past history of specific diseases except the recent stoke. The initial ECG obtained after transfer showed bradycardia (55 beats/min), PR prolongation followed by AV block characteristic of second degree AV block, Mobitz type 1 with left axis deviation and poor R wave progression (Fig. 2A). On admission, the patient's vital signs were as follows: blood pressure, 120/80 mmHg; heart rate, 49 beats/min; respiratory rate, 20 breaths/min; temperature, 36.7℃. He had white blood cell count 8020×109/L (neutrophil 68.5%); hemoglobin, 15.1 g/dL; platelet count, 202000/L; and serum C-reactive protein, 0.42 mg/dL. Additionally, serum electrolyte, serum creatine kinase-myocardial band, serum troponin I level and N-terminal pro B-type natriuretic peptide were within normal range. There was no specific finding in the chest x-ray. Holter monitoring showed high grade AV block combined with 2:1 AV block (Fig. 2B and C). Treadmill test was performed for the evaluation of a chronotrophic response; however, the patient's maximum heart rate observed during Bruce protocol stage 3 was 117 beats/min without reaching the target heart rate of 163 beat/min. Transthoracic echocardiography demonstrated a loss of A wave mimicking atrial fibrillation with peak E wave velocity of 59.72 cm/sec and deceleration time of 183 msec. Global left ventricular (LV) ejection fraction was 67% with E/E' of 5.24 implying normal LV systolic function and filling pressure. End diastolic diameters of each ventricle were within normal ranges i.e. 51.80 mm on the left and 33.10 mm on the right ventricle. Interestingly, unsuspected heavy trabeculations in the LV apex were observed (Fig. 3A). We performed contrast echocardiography and cardiac MRI for a more definite diagnosis. Contrast echocardiography showed flow communication between multiple intertrabecular recesses and LV cavity with noncompacted/compacted ratio of approximately 3:1 (Fig. 3B). Cardiac MRI demonstrated prominent trabeculations and deep intertrabecular recesses with thinner compact epicardial layer of the LV characteristic of LVNC (Fig. 4). Mild spontaneous echo contrast in LA appendage without definite thrombi and decreased mean LA appendage flow velocity of 19.93 cm/sec were observed on transesophageal echocardiography with no definite atherosclerotic change from the aortic arch to thoracic aorta. Eletrophysiologic (EP) test was performed to evaluate the cause of arrhythmia and to determine pacemaker insertion. Prolongation of ventricular interval was documented (87 msec) (Fig. 5A); interestingly, although there was 1:1 AV relationship during pacing from high right atrium (RA), there was no atrial signal on coronary sinus (CS) bipole with no correlation between atrial and ventricular signal while pacing from RA appendage that implied absence of electrical activity of LA on EP study (Fig. 5B). Capture failure was demonstrated during the pacing of septum portion of RA and LA. For further corroboration of the results, we carried out an additional EP study using 3 dimensional voltage mapping (Fig. 6). The map showed no voltage signal of the entire LA, septum and body of RA, which explained the capture failure during pacing of RA septum and LA. Based on the prior results, we decided to initiate warfarinzation keeping in mind permanent pacemaker insertion; however, we could not perform the procedure due to the patients' refusal. He was discharged from hospital and remained asymptomatic without further complication until the latest follow up visit.
We reported a case of a young male patient with concomitant LV noncompaction and LA standstill that has not been reported before. The etiology of CVA in this case can be explained by 2 conditions: firstly, the noncompaction itself as a complication; and secondly, low velocity in the LA appendage. Treatment was decided based on the 2 positive findings during patient evaluation.
LVNC was first described as "spongy myocardium" in 1975 by Dusek et al.4) and is characterized by morphological abnormality of prominent trabeculae representing "persistent sinusoids" and myocardial wall thickening; the new term "isolated non-compaction of LV myocardium" was introduced by Chin et al.5) in 1990 emphasizing deep recesses communicating with the cavity of LV and absence of other cardiac deformities. Although it is defined as a primary cardiomyopathy by the American Heart Association, it still remains as a "unclassified" cardiomyopathy according to the World Health Organization.6) The etiologies of isolated LVNC are yet obscure, though several genetic mechanisms that might be involved in the arrest of myocardial development during embryogenesis were recently suggested.3)
The various clinical manifestations of LVNC reportedly include congestive heart failure, systemic embolic events, and arrhythmias. The degree of symptoms usually depends on the grade of noncompaction and ventricular function.3)
Two representative methods used for the diagnosis of LVNC are echocardiography and cardiovascular magnetic resonance (CMR). Generally, the diagnosis of LVNC largely depends on 2 dimensional/3 dimensional echocardiography, with or without contrast echocardiography due to cost-effectiveness. We applied 3 different echocardiographic diagnostic criteria by Chin et al.,5) Jenni et al.,7) and Stöllberger et al.8) based on parameters of ventricular morphology, although there is no officially established diagnostic criterion; therefore, multimodal imaging approach inevitably improves diagnostic accuracy. In case of low echocardiographic image quality, CMR shows better signal to noise ratio, contrast to noise ratio, unlimited imaging planes and ability to use tissue characterization. There are 2 CMR criteria proposed by Petersen et al.9) and Jacquier et al.,10) either using the ratio of noncompact/compact myocardium depth (>2.3) or trabecular mass (>20%).
Management of LVNC focuses on standard medical therapy for systolic and diastolic ventricular dysfunction and prevention of systemic embolic events.3) We applied warfarin for the prevention and treatment of CVA. There is controversy regarding cardioverter-defibrillator (implantable cardioverter-defibrillator) implantation in patients with LVNC but should be considered as primary prevention in high risk patients; alternatively, cardiac transplantation can be considered in these patients.11) Once LVNC is diagnosed, family screening of first degree relatives is recommended.3)
The prognosis of LVNC is still controversial. Initial reports showed very poor prognosis in patients with LVNC; however, recent studies supported a better prognosis.6)12) One study showed 15% mortality among LVNC patients during a mean 30 month follow-up.12)
Previously reviewed studies and case reports indicate various ECG findings among the LVNC patients; for example, normal ECG, atrial fibrillation, bundle branch block, QT interval prolongation, left ventricular hypertrophy, AV block, and repolarization abnormality.13) There were several cases of both atrial standstill in LVNC patients; however, our patient showed isolated LA standstill that has not been reported previously in LVNC patients. Atrial standstill is an uncommon arrthymia characterized by the absence of electrical and mechanical activity in the atrium.14) On EGC, atrial standstill is distinguished by the absence of P waves with bradycardia and narrow junctional escape rhythm.14) Known etiologies of atrial standstill are electrolyte imbalance, familial cause, cardiomyopathies, valvular disease, muscular dystrophy and toxicity of antiarrhythmic agents.15) Several types of atrial standstill were previously described; the more frequent transient type, the rare persistent type and total or partial types.15) Recently, familial atrial standstill was reported in several studies showing tendency of sudden cardiac death with higher probability of cardiomyopathies at a young age. The genetic causes of familial type are still obscure; however, cardiac sodium channel gene SCN5A was identified as a related gene mutation.16) The study patient showed features of isolated LA standstill demonstrating electrical silence in the LA that is isolated from partially electrically active RA. Isolated LA standstill is extremely rare; it has previously been reported in severe mitral valvular stenosis, dilated cardiomyopathy or following LA ablation.15)
However, none of the possible factors was found in our patient, thus raising the question whether the abnormal ECG finding was correlated with LVNC or occurred independently. The EP study demonstrated complete electrical block between RA and LA, showing no atrial conduction impulse in the CS bipole with no electrical activity in LA, which supported LA thrombus formation as the etiology of CVA.
In conclusion, both LVNC and LA standstill have risks of embolic events. While the connection between these 2 conditions is still ambiguous, coagulation is a certainty. Here we reported a case of a young man who had LVNC combined with LA standstill with CVA sequel without any cardiac symptoms.
Figures and Tables
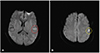 | Fig. 1Local brain MRI showing focal high signal intensity lesions in the left temporal lobe (A. red circle) and left frontal lobe (B. yellow circle) on diffusion weighted image. MRI: magneticresonance imaging. |
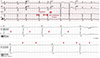 | Fig. 2(A) Electrocardiogram showing bradycardia (55 beats/min), PR prolongation followed by AV block demonstrating characteristic of second degree AV block, Mobitz type 1, left axis deviation and poor R wave progression. (B and C; red arrows mean P wave) 24 hour Holter monitoring showing intermittent 2:1 AV block with high grade AV block. AV: atrioventricular. |
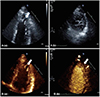 | Fig. 3(A) Transthoracic echocardiography (TTE). A-(a): Arrow shows heavy trabeculations in LV apex. A-(b): Due to trabeculations, myocardium of LV apex has a spongy appearance on the short axis view of TTE. (B) Contrast echocardiography. B-(a): Arrow shows heavy trabeculations in heavy trabeculations in LV apex is clearly observed with noncompacted/compacted ratio of approximately 3:1. B-(b): Arrow shows communicating flow in multiple intertrabecular recesses with LV cavity. LV: left ventricle. |
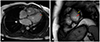 | Fig. 4Cardiac MRI showing prominent trabeculations and deep intertrabecular recesses (red line) with thinner compact epicardial side (yellow line) with noncompacted/compacted ratio of approximately 3:1. MRI: magnetic resonance imaging, RV: right ventricle, LV: left ventricle. |
 | Fig. 5Electrophysiologic test. A: Study demonstrates prolongation of HV interval of 87 msec. B-(a): Study showing 1:1 AV relationship during pacing from high RA. B-(b): Study demonstrating no atrial signal on CS bipole with no correlation between atrial and ventricular signal while pacing from RA appendage. HV: his-ventricular, AV: atrioventricular, RA: right atrium, CS: coronary sinus. |
References
1. Thuny F, Jacquier A, Jop B, et al. Assessment of left ventricular noncompaction in adults: side-by-side comparison of cardiac magnetic resonance imaging with echocardiography. Arch Cardiovasc Dis. 2010; 103:150–159.
2. Pitta S, Thatai D, Afonso L. Thromboembolic complications of left ventricular noncompaction: case report and brief review of the literature. J Clin Ultrasound. 2007; 35:465–468.
3. Weiford BC, Subbarao VD, Mulhern KM. Noncompaction of the ventricular myocardium. Circulation. 2004; 109:2965–2971.
4. Dusek J, Ostádal B, Duskova M. Postnatal persistence of spongy myocardium with embryonic blood supply. Arch Pathol. 1975; 99:312–317.
5. Chin TK, Perloff JK, Williams RG, Jue K, Mohrmann R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation. 1990; 82:507–513.
6. Habib G, Charron P, Eicher JC, et al. Isolated left ventricular noncompaction in adults: clinical and echocardiographic features in 105 patients. Results from a French registry. Eur J Heart Fail. 2011; 13:177–185.
7. Jenni R, Oechslin E, Schneider J, Attenhofer Jost C, Kaufmann PA. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: a step towards classification as a distinct cardiomyopathy. Heart. 2001; 86:666–671.
8. Stöllberger C, Finsterer J, Blazek G. Left ventricular hypertrabeculation/noncompaction and association with additional cardiac abnormalities and neuromuscular disorders. Am J Cardiol. 2002; 90:899–902.
9. Petersen SE, Selvanayagam JB, Wiesmann F, et al. Left ventricular non-compaction: insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol. 2005; 46:101–105.
10. Jacquier A, Thuny F, Jop B, et al. Measurement of trabeculated left ventricular mass using cardiac magnetic resonance imaging in the diagnosis of left ventricular non-compaction. Eur Heart J. 2010; 31:1098–1104.
11. Stanton C, Bruce C, Connolly H, et al. Isolated left ventricular noncompaction syndrome. Am J Cardiol. 2009; 104:1135–1138.
12. Aras D, Tufekcioglu O, Ergun K, et al. Clinical features of isolated ventricular noncompaction in adults long-term clinical course, echocardiographic properties, and predictors of left ventricular failure. J Card Fail. 2006; 12:726–733.
13. Steffel J, Kobza R, Oechslin E, Jenni R, Duru F. Electrocardiographic characteristics at Initial diagnosis in patients with isolated left ventricular noncompaction. Am J Cardiol. 2009; 104:984–989.
14. Groenewegen WA, Firouzi M, Bezzina CR, et al. A cardiac sodium channel mutation cosegregates with a rare connexin40 genotype in familial atrial standstill. Circ Res. 2003; 92:14–22.
15. Duncan E, Schilling RJ, Earley M. Isolated left atrial standstill identified during catheter ablation. Pacing Clin Electrophysiol. 2013; 36:e120–e124.
16. Fazelifar AF, Arya A, Haghjoo M, Sadr-Ameli MA. Familial atrial standstill in association with dilated cardiomyopathy. Pacing Clin Electrophysiol. 2005; 28:1005–1008.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download