Abstract
Background and Objectives
Frequent ventricular premature complexes (VPCs) increase the risk of cardiomyopathy (CMP). However, most data regarding VPCs have been obtained from Western population and in-hospital patient-based studies. The objective of this study was to define the clinical characteristics and features of idiopathic VPCs in the Korean population.
Subjects and Methods
We investigated subjects undergoing transthoracic echocardiography and documented VPC burdens >1% by Holter monitoring in an outpatient clinic at Samsung Medical Center, Korea. We analyzed demographic and clinical features and the nature of the VPCs by electrocardiography (ECG).
Results
A total of 666 patients were registered. Mean age was 54.7±16.8 years, and 365 (54.8%) patients were female. Typical VPC-related symptoms, such as palpitation and a dropped beat, were observed in 394 (59.2%) patients. Some patients received beta-blockers (n=95; 14.3%) and anti-arrhythmic agents (n=14; 2.1%). The ECG analysis was performed in 405 patients; 322 (79.5%) exhibited left bundle branch block (LBBB) and 347 (85.8%) exhibited an inferior axis. The precordial R-wave transition was predominantly distributed over V3 in 230 patients (56.6%). Thirty-one patients (4.5%) were diagnosed with VPC-induced CMP.
Ventricular premature complexes (VPCs) are frequently observed on 12-lead electrocardiography (ECG) in healthy populations and in patients with ischemic/structural heart disease.1) According to a population-based study in the United States, >6% of middle-aged adults have VPCs, and prevalence increases with age.1)2) Accumulating evidence suggests that frequent VPCs are a possible cause of sudden cardiac death and reversible cardiomyopathy (CMP) in the general population.1)3)4)5) However, most of the data on the characteristics and features of VPCs have been obtained from Western population and in-hospital patient-based studies. The aim of this study was to define the clinical characteristics and features of idiopathic VPCs in the Korean population. We focused on outpatient clinic patients in a single center and analyzed the clinical and electrocardiographic characteristics of patients with frequent idiopathic VPCs.
A total of 2341 patients diagnosed with frequent VPCs in the outpatient clinic regardless of the reason for their visit to Samsung Medical Center from January 1994 to December 2013 were included in a retrospective, single-center VPC registry. Among them, 666 patients were finally enrolled in this study according to the following inclusion criteria (Fig. 1): 1) frequent VPCs (>1% or >1000 beats/day) on 24-hr Holter ECG (SEER Light Extend Compact Holter Recorders, GE Medical Systems, Fairfield, Conn., USA) monitoring at enrollment, 2) symptoms fully described in medical records, and 3) underwent baseline and follow-up echocardiography within 6 months from enrollment. Exclusion criteria were: 1) history of atrial fibrillation, atrial flutter, atrial tachycardia, non-sustained ventricular tachycardia, and sustained ventricular tachycardia, or documented arrhythmias by 12-lead ECG (PageWriter TC30, Philips Medical Systems, Amsterdam, Netherlands) or Holter ECG monitoring, 2) history of myocardial infarction, structural heart disease, or heart valve replacement/repair, and 3) any evidence of ischemic/structural heart disease based on echocardiography, a radionuclide evaluation, and/or cardiac catheterization. All transthoracic echocardiography (TTE) data and Holter monitoring data were reviewed. Symptoms related to VPCs were evaluated by a cardiologist based on the patient's medical records. Palpitation and dropped beats were regarded as typical VPC-related symptoms, and all other symptoms, such as fatigue, dizziness, syncope, and shortness of breath, were defined as atypical symptoms. The ECG analysis was performed on 405 patients with ECG containing VPC and taken anytime during the follow up period. All procedures were performed following the institutional guidelines of Samsung Medical Center, and all patients provided their written informed consent.
TTE was performed with subjects in the left lateral decubitus position. Left ventricular (LV) systolic function was measured using the modified Simpson's method (biplane method) according to the recent American Society of Echocardiography committee recommendations.6) Normal LV systolic function was defined as ejection fraction (EF) ≥50% based on American College of Cardiology Foundation (ACCF)/American Heart Association (AHA) guidelines.7) According to this definition, EF<50% was classified as LV systolic dysfunction. In addition, TTE and a quantitative assessment of LV function was repeated at 3-6 month intervals in patients with LV dysfunction.
Patients available for a 12-lead ECG assay were included in this sub-group. The initial ECG taken during the study period was the criterion. We investigated the pattern and axis of VPCs, and the distributions of the precordial R wave transition. The parameters were defined as follows: 1) VPC patterns: left bundle branch block (LBBB) and right bundle branch block (RBBB) were defined in accordance with recent ACCF/AHA/Heart Rhythm Society recommendations;8) 2) VPC axes: positive and negative axes were determined by the vector of the dominant VPC deflection in leads II, III, and aVF; 3) precordial R wave transition: the R-wave transition at leads V1-V2 was defined as below V3, and a transition at leads V4-V6 was defined as above V3.
Holter monitoring was performed before treatment to determine VPC burden. Follow-up Holter monitoring was repeated 3-6 months after treatment (radiofrequency ablation or anti-arrhythmic drugs) and again if palpitations recurred.
Continuous variables are presented as mean±standard deviation, and differences were assessed with the independent t-test or the Wilcoxon rank-sum test. Categorical variables are described as numbers (n) with percentages (%), and differences were analyzed with Pearson χ2 or Fisher's exact test. A p-value <0.05 was considered significant. All statistical analyses were conducted with two-tailed tests using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA).
A total of 666 patients were registered in this study. Age was normally distributed (Fig. 2); 40-50 year old females were dominant (65.2%), whereas most males were 50-70 years old (61.1%). Of the patients, 365 were female (54.8%), and their mean age was 54.79±16.88 years (Table 1). Overall, 499 (74.9%) patients were never-smokers, 89 (13.4%) were ex-smokers, and 78 (11.7%) were current smokers. The regional distribution of the study population was as follows: Seoul, 334 (50.2%); Gyeonggi-do, 185 (27.8%); Chungcheongnam-do, 25 (3.8%); Gyeongsangbuk-do, 20 (3.0%); and Chungcheongbuk-do, 12 (1.8%) (Fig. 3). In this study population, 56 (8.4%) patients had diabetes, 157 (23.6%) had hypertension, and 17 (2.6%) had dyslipidemia. They were prescribed a number of medications, such as beta-blockers (95 patients; 14.3%), calcium channel blockers (42 patients; 6.3%), angiotensin-converting enzyme inhibitors (11 patients; 1.7%), angiotensin II receptor blockers (33 patients; 5.0%), and anti-arrhythmic agents (14 patients; 2.1%). Symptoms ranged from typical VPC-related symptoms, such as palpitation and dropped beats (394 patients; 59.2%), to atypical symptoms, such as chest pain (218 patients; 32.7%), dizziness (109 patients; 16.4%), syncope (36 patients; 5.4%), and fatigue (11 patients; 1.7%). LV systolic function was preserved in 635 (95.3%) patients, and 31 (4.7%) patients demonstrated LV dysfunction on TTE. The VPC burden as observed by Holter monitoring was 11.81±10.05%. We compared these epidemiological and clinical characteristics based on LV systolic function (Table 2). The median interval between the day of Holter ECG and the day of TTE was 29.5 months (interquartile range [IQR], 3.8-59.6 months). LV dysfunction was observed more frequently in males than in female using TTE (p=0.027). Age, height, weight, residential district, smoking status, combined medical conditions, and prescribed medication were not significantly different between the groups. Female patients were more sensitive to typical VPC-related symptoms than males (odds ratio, 1.83, 95% confidence interval, 1.34-2.51; p<0.01).
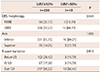
A total of 405 patients were included in the ECG sub-group analysis (Table 3). The LBBB pattern of VPCs was seen in 322 patients (79.5%), and the RBBB pattern was seen in 83 patients (20.5%). Of the patients given an ECG, 347 (85.8%) exhibited VPCs with an inferior axis, and 58 patients (14.2%) exhibited a superior axis. R transition points were divided into three positions of below V3 in 105 patients (26.0%), at V3 in 70 patients (17.4%), and over V3 in 230 patients (56.6%). No differences were found when we compared the VPC characteristics based on LV systolic function (Table 4).
The median follow-up duration was 3.5 years (IQR, 0.4-6.0 years). During the follow up, thirty-one patients developed LV dysfunction and 30 patients died during the follow-up period. The cause of the deaths were cancer in 23 patients, progression of heart failure in two, acute myocardial infarction in one, sudden cardiac death in one, and other causes in three patients.
This is the first study to investigate the demographic and clinical characteristics of VPCs in a Korean population. The major findings were that frequent VPCs were slightly more prevalent in females; however, the incidence of LV dysfunction was higher in males and patients without typical VPC-related symptoms. Some studies have suggested that VPCs are associated with an increase in all-cause mortality, myocardial infarction, and cardiac death.9) However, these results have been criticized for overlooking the potential confounding effect of underlying heart disease. Our study was conducted with patients from an outpatient clinic at a single center. We excluded ischemic or structural heart disease and tachyarrhythmia, which may cause tachycardia-induced CMP. Although patients visit our hospital for various reasons, we made an effort to confine the study population to the general population with idiopathic VPCs.
VPCs are more usually prevalent among males than females, and the prevalence of VPCs increases with age.1)2) Therefore, it is more reasonable to demonstrate a crescendo pattern in the age distribution because of the cumulative effect. However, we found that idiopathic VPCs were more prevalent in females and that age was normally distributed. Moreover, the most prevalent age among females was a decade younger than that of males. We assumed that because we excluded patients with ischemic or valvular heart disease that older male patients were relatively less represented (Supplementary Fig. 1 in the online-only Data Supplement); this may have caused the difference between our study results and those of previous studies. When we compared the presence of typical VPC-related symptoms in both sexes, females were more sensitive to VPCs. Many studies have demonstrated sexual differences between patients with normal LV function and those with CMP, and the incidence of VPC-induced CMP is higher in males than in females.10)11)12)13) Male gender is a major risk factor for coronary heart disease,14) and VPCs are observed more frequently in patients with ischemic heart disease.15) These associations may have affected the outcomes of previous studies. However, when we excluded ischemic factors, sex-based differences in sensitivity to VPCs may be a more reasonable explanation for our study results.
The most common ECG features of VPCs in this study were LBBB, inferior axis, and late precordial R-wave transition. The main origin of idiopathic VPCs was the right ventricular outflow tract.16)17)18)19) Significant accumulated evidence indicates that right ventricular outflow tract origin-VPCs manifest the LBBB pattern, inferior axis, and precordial R-wave transition at or over V3.19)20)21)22) Based on these results, we assumed that VPCs in the Korean population predominantly originated from the right ventricular outflow tract, and this result is consistent with previous studies. A retrospective study demonstrated that the distribution of the site of origin is significantly different between normal LV function and CMP groups, and that non-outflow tract sites of origin were more prevalent in the CMP group.23) However, that study population was limited to catheter ablation candidates. In our study of the general population, the origin of VPCs was similar between the normal LV function and LV dysfunction groups.
We cannot explain the relationship between VPC burden and CMP in this study because this was a retrospective study. Thus, we could not follow-up changes in LV function and VPC burden periodically in all patients. Another reason is that the examination times for ECG, Holter monitoring, and the TTE study were different, so we cannot explain the relationship between LV function and the VPCs features. The clinical basis for the noted differences associated with LV dysfunction in this study deserves a further prospective study.
This study had several limitations. First, the results may have been significantly affected by unrecognized confounding factors due to its retrospective nature. Second, we used surface ECG to interpret the VPC characteristics, which may have led to limitations regarding ECG lead position, cardiac rotation, and respiratory variations. However, the consistency of our results with those of previous studies confirms the reliability of outcomes despite missing data and the limitation of surface 12-lead ECG. Finally, there is a lack of follow-up data. The median interval between the day of Holter ECG and the day of TTE was 518 days. Despite these limitations, we revealed the clinical and electrocardiographic characteristics of VPCs in a Korean population. We strictly limited the study population to individuals with "normal" hearts, and excluded tachyarrhythmias that cause LV dysfunction other than idiopathic VPCs. In contrast to previous studies, including patients with high VPC burdens who were slated to undergo catheter ablation, we reduced selection bias to target patients referred from an outpatient clinic.
Figures and Tables
Fig. 1
Study scheme. Search flow diagram of the study population. VPCs: ventricular premature complexes, AF: atrial fibrillation, AFL: atrial flutter, AT: atrial tachycardia, NSVT: non-sustained ventricular tachycardia, SVT: sustained ventricular tachycardia, IHD: ischemic heart disease, SHD: structural heart disease, LVEF: left ventricular ejection fraction, ECG: electrocardiography.
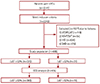
Fig. 2
Age distribution of the study population. The prevalence of ventricular premature complexes was slightly higher in females than that in males, particularly in 40-60 year old women. The peak age for females was 50-70 years; however the peak age for males was 60-80 years. Age was normally distributed in the study population.

Fig. 3
Regional distribution of study population on the Korean peninsula. The regional distribution of the study population was: Seoul 334 (50.2%), Gyeonggi-do 185 (27.8%), Chungcheongnam-do 25 (3.8%), Gyeongsangbuk-do 20 (3.0%), Gangwon-do 12 (1.8%), Chungcheongbuk-do 12 (1.8%), Daejeon 11 (1.7%), Daegu 11 (1.7%), Busan 10 (1.5%), Jeollanam-do 9(1.4%), Gyeongsangnam-do 9 (1.4%), Incheon 7 (1.1%), Gwangju 7 (1.1%), Jeollabuk-do 5 (0.8%), Jeju 4 (0.6%), and Ulsan 3 (0.5%). The regional distribution of ventricular premature complex-induced cardiomyopathy on the Korean peninsula was not significant (p=0.510).
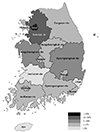
Table 1
Demographic and clinical characteristics of the study population

Table 2
Demographic and clinical characteristics based on left ventricular systolic function
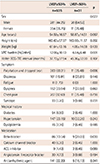
Table 3
Electrocardiographic characteristics of the study population

| Variables | n=405 |
|---|---|
| QRS morphology | |
| RBBB | 83 (20.5) |
| LBBB | 322 (79.5) |
| Axis | |
| Inferior | 347 (85.8) |
| Superior | 58 (14.2) |
| R wave transition | |
| Below V3 | 105 (26.0) |
| At V3 | 70 (17.4) |
| Over V3 | 230 (56.6) |
Table 4
Electrocardiographic analysis and comparison based on left ventricular systolic function
Acknowledgments
This research was supported by a grant from the Korean Society of Cardiology (2012).
References
1. Cheriyath P, He F, Peters I, et al. Relation of atrial and/or ventricular premature complexes on a two-minute rhythm strip to the risk of sudden cardiac death (the Atherosclerosis Risk in Communities [ARIC] study). Am J Cardiol. 2011; 107:151–155.
2. Simpson RJ Jr, Cascio WE, Schreiner PJ, Crow RS, Rautaharju PM, Heiss G. Prevalence of premature ventricular contractions in a population of African American and white men and women: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2002; 143:535–540.
3. Chugh SS, Shen WK, Luria DM, Smith HC. First evidence of premature ventricular complex-induced cardiomyopathy: a potentially reversible cause of heart failure. J Cardiovasc Electrophysiol. 2000; 11:328–329.
4. Ataklte F, Erqou S, Laukkanen J, Kaptoge S. Meta-analysis of ventricular premature complexes and their relation to cardiac mortality in general populations. Am J Cardiol. 2013; 112:1263–1270.
5. Bogun F, Crawford T, Reich S, et al. Radiofrequency ablation of frequent, idiopathic premature ventricular complexes: comparison with a control group without intervention. Heart Rhythm. 2007; 4:863–867.
6. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005; 18:1440–1463.
7. Writing Committee members. Yancy CW, Jessup M, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013; 128:e240–e327.
8. Hancock EW, Deal BJ, Mirvis DM, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009; 53:992–1002.
9. Ng GA. Treating patients with ventricular ectopic beats. Heart. 2006; 92:1707–1712.
10. Del Carpio Munoz F, Syed FF, Noheria A, et al. Characteristics of premature ventricular complexes as correlates of reduced left ventricular systolic function: study of the burden, duration, coupling interval, morphology and site of origin of PVCs. J Cardiovasc Electrophysiol. 2011; 22:791–798.
11. Baman TS, Lange DC, Ilg KJ, et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm. 2010; 7:865–869.
12. Yokokawa M, Kim HM, Good E, et al. Impact of QRS duration of frequent premature ventricular complexes on the development of cardiomyopathy. Heart Rhythm. 2012; 9:1460–1464.
13. Ban JE, Park HC, Park JS, et al. Electrocardiographic and electrophysiological characteristics of premature ventricular complexes associated with left ventricular dysfunction in patients without structural heart disease. Europace. 2013; 15:735–741.
14. Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998; 97:1837–1847.
15. Kostis JB, Byington R, Friedman LM, Goldstein S, Furberg C. Prognostic significance of ventricular ectopic activity in survivors of acute myocardial infarction. J Am Coll Cardiol. 1987; 10:231–242.
16. Movsowitz C, Schwartzman D, Callans DJ, et al. Idiopathic right ventricular outflow tract tachycardia: narrowing the anatomic location for successful ablation. Am Heart J. 1996; 131:930–936.
17. Kamakura S, Shimizu W, Matsuo K, et al. Localization of optimal ablation site of idiopathic ventricular tachycardia from right and left ventricular outflow tract by body surface ECG. Circulation. 1998; 98:1525–1533.
18. Kanagaratnam L, Tomassoni G, Schweikert R, et al. Ventricular tachycardias arising from the aortic sinus of valsalva: an under-recognized variant of left outflow tract ventricular tachycardia. J Am Coll Cardiol. 2001; 37:1408–1414.
19. Dixit S, Gerstenfeld EP, Callans DJ, Marchlinski FE. Electrocardiographic patterns of superior right ventricular outflow tract tachycardias: distinguishing septal and free-wall sites of origin. J Cardiovasc Electrophysiol. 2003; 14:1–7.
20. Callans DJ, Menz V, Schwartzman D, Gottlieb CD, Marchlinski FE. Repetitive monomorphic tachycardia from the left ventricular outflow tract: electrocardiographic patterns consistent with a left ventricular site of origin. J Am Coll Cardiol. 1997; 29:1023–1027.
21. Lin D, Ilkhanoff L, Gerstenfeld E, et al. Twelve-lead electrocardiographic characteristics of the aortic cusp region guided by intracardiac echocardiography and electroanatomic mapping. Heart Rhythm. 2008; 5:663–669.
22. Betensky BP, Park RE, Marchlinski FE, et al. The V(2) transition ratio: a new electrocardiographic criterion for distinguishing left from right ventricular outflow tract tachycardia origin. J Am Coll Cardiol. 2011; 57:2255–2262.
23. Carballeira Pol L, Deyell MW, Frankel DS, et al. Ventricular premature depolarization QRS duration as a new marker of risk for the development of ventricular premature depolarization-induced cardiomyopathy. Heart Rhythm. 2014; 11:299–306.
Supplementary Material
The online-only Data Supplement is available with this article at http://dx.doi.org/10.4070/kcj.2015.45.5.391.
Supplementary Fig. 1
Age and sex distribution of the entire registry based on the inclusion and exclusion criteria. The percentage of excluded patients, particular males, increased with age after their 40s. The percentage of patients excluded who were <40 years decreased with age. The overall percentage of excluded patients showed a J-shaped curve reaching a nadir at the fourth decade on the age distribution graph. M: male, F: female.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download