Abstract
Background and Objectives
The red blood cell distribution width (RDW) has been found to be associated with cardiovascular morbidity and mortality. The objective of this study was to determine whether the RDW measures are associated with the coronary artery calcification score (CACS) in patients who did not present with obvious coronary heart disease (CHD).
Subjects and Methods
A total of 527 consecutive patients with a low to intermediate risk for CHD but without obvious disease were enrolled in this study. The study subjects underwent coronary computerized tomography angiography and CACS was calculated. The patients were divided into two groups based on CACS: Group I (CACS≤100) and Group II (CACS>100). The two groups were compared in terms of classic CHD risk factors and haematological parameters, particularly the RDW.
Results
Group I patients were younger than Group II patients. The Framingham risk score (FRS) in patients of Group II was significantly higher than that in patients of Group I. Group II patients had significantly elevated levels of haemoglobin, RDW, neutrophil count, and neutrophil/lymphocyte ratio compared with Group I patients. CACS was correlated with age, RDW, and ejection fraction. In the multivariate analysis, age, RDW, and FRS were independent predictors of CACS. Using the receiver-operating characteristic curve analysis, a RDW value of 13.05% was identified as the best cut-off for predicting the severity of CACS (>100) (area under the curve=0.706).
The red blood cell distribution width (RDW), part of the standard complete blood count, is a measure of the variability in circulating erythrocyte size that is often used in the differential diagnosis of anaemia.1) Recently, the RDW was found to be associated with a poor prognosis and increased mortality rate in several cardiovascular diseases such as stable coronary heart disease,2) heart failure,3) and peripheral artery disease,4) and also with some invasive cardiac procedures such as primary coronary intervention5) and transcatheter aortic valve implantation.6) The RDW is also elevated in some subclinical states of atherosclerosis, e.g., in patients with microalbuminuria, which is an independent predictor of cardiovascular (CV) events.7) Computerized tomography (CT) can be used to calculate the coronary artery calcification score (CACS), which is a measure of the plaque burden in the coronary arteries. The severity of coronary artery calcification (CAC) has been found to be a useful marker of atherosclerosis,8) and is an independent predictor of CV events.9) Although the relationship between the RDW and CV disease (CVD) is well-documented in the literature, there are limited data concerning the relationship between haematological parameters and the CACS.10) The goal of this study was to determine whether serum RDW measures are associated with the CACS in patients with a low to intermediate risk for coronary heart disease (CHD) but without known CHD.
We enrolled 527 consecutive outpatients who had no exclusion criteria and no obvious CHD, but who were found to have a low to intermediate risk for CHD based on their Framingham risk score (FRS) as described by Wilson et al.11)
Patients were excluded from the study if they had evidence of coronary artery or cerebrovascular disease, heart failure, renal or hepatic dysfunction, a history of cancer, systemic inflammatory disease, autoimmune disease, any haematological system disorder, thyroid disease, or a recent infection (within the previous three months). Baseline characteristics of patients, including age, sex, presence of hypertension (HT) and/or diabetes mellitus (DM), smoking status, left ventricular ejection fraction, FRS, complete blood count parameters, uric acid, fasting serum lipid levels, and C-reactive protein levels were recorded and compared according to the CACS. All of the patients underwent coronary CT angiography and the CACS was calculated.
The study protocols were approved by the local ethics committee, and they complied with the Declaration of Helsinki.
Venous blood samples were obtained following a 12-hour fasting period and used in all laboratory tests. The lipid profile in each patient was measured by standard methods. Haematological indices were measured using an automated blood cell counter (Beckman Coulter HmX AL Haematology Analyzer, Brea, CA, USA).
All CT scans were performed using a 64-slice scanner (Philips Brilliance 64, Philips Medical Systems, Eindhoven, Netherlands) with a 0.42-second rotation time with a pitch of 0.2, a tube voltage of 120 kVp, and a tube current of 600-1050 mAs. Patients with heart rates >70 beats/minute were treated with metoprolol intravenously (5 to 15 mg) or orally (100 mg) one hour before the scan unless they had any known contraindications for beta-blocker usage. All data sets were reconstructed using retrospective electrocardiographic gating at 40%, 75%, and 80% of the inter-beat (RR) interval. Data sets were reconstructed using a dedicated workstation (EBW, Philips Medical Systems, Highland Heights, OH, USA). The calcium score for each artery was calculated as the sum of calcium scores in the left main, left anterior descending, left circumflex, and right coronary arteries according to the Agatston method.12)
The SPSS 20.0 software package (SSPS, Inc., Chicago, IL, USA) was used for statistical analyses. Continuous variables were expressed as means±standard deviation if they were normally distributed, or as medians with interquartile ranges if they were not normally distributed. Categorical variables were expressed as total number (percentage). All continuous variables were checked with the Kolmogorov-Smirnov normality test to assess their distribution pattern. Normally distributed variables were compared using the unpaired Student t test and analysis of variance (ANOVA), while abnormally distributed variables were compared using the Mann-Whitney U test and ANOVA. For categorical variables, the chi-square test was used. Pearson and Spearman correlation coefficients were used to calculate univariate correlations. Multiple linear regression analysis with forward stepwise selection was performed to identify factors independently associated with the CACS. Receiver-operating characteristic (ROC) curves for RDW values were used to determine the optimal cut-off values in order to predict the severity of CAC and to establish the optimal cut-off points for use in clinical decision-making. A p value <0.05 was considered statistically significant.
A total of 527 consecutive patients were enrolled in this study. The median age of patients was 56 years (range; 34-81 years), and 380 (72.1%) patients were males.
On the basis of the CACS, patients were divided into two groups: Group I (CACS≤100) and Group II (CACS>100). Table 1 summarizes the demographic, clinical, and laboratory data of the two groups. The mean age of Group I patients was significantly lower than the mean age of Group II patients {55.0 years (range, 34-73 years) vs. 58.5 years (range, 48-81 years), p<0.001}. The rates of DM and HT were significantly higher in Group II than in Group I (15.1% vs. 10.7%, p=0.001 and 67.0% vs. 51.3%, p=0.006, respectively).
Compared with Group I patients, Group II patients had significantly higher FRS (11.9±6.2 vs. 7.4±5.6, p<0.001), haemoglobin (14.80±1.47 vs.14.71±1.51, p=0.03), RDW {12.97 (1.1) vs. 12.80 (1.0), p=0.01}, neutrophil count (1.82±0.98 vs. 1.60±1.08, p=0.01), and neutrophil/lymphocyte ratio (NLR) {1.54 (1.0) vs. 1.39 (0.6), p=0.008}. The between-group differences in RDW and NLR are shown in Fig. 1.
The bivariate correlation analysis revealed that the CACS was positively correlated with patient age (r=0.386, p<0.001), HT (r=0.227, p<0.001), DM (r=0.148, p=0.007), FRS (r=0.271, p<0.001), and RDW (r=0.202, p<0.001) (Fig. 2), and it was inversely correlated with the ejection fraction (r=-0.122, p=0.01).
The results of multivariate analysis showed that patient age (β=0.300, p<0.001), RDW (β=0.156, p=0.001), and FRS (β=0.119, p=0.02) were significant independent predictors of the CACS (Table 2).
Using the ROC curve analysis, an RDW value of 13.05% was identified as an effective cut-off point for predicting the severity of the CACS (>100), with a sensitivity of 58.9% and a specificity of 72.0%, and the area under the curve (AUC) was 0.706 (Fig. 3).
The present study demonstrated that a CACS greater than 100 is associated with higher patient age, DM, HT, FRS, Hb, RDW, neutrophil count, and NLR. In addition, the patient age, RDW, and FRS were found to be independent predictors of the CACS. An RDW value of 13.05% was identified as an effective cut-off point for predicting the severity of the CACS (>100) with a sensitivity of 58.9% and a specificity of 72.0% (AUC=0.706).
The association between the presence of CAC and the risk of cardiovascular events has been established for several years.13) In addition to the traditional risk factors,14)15) the CACS has prognostic value in asymptomatic patients and may have an incremental benefit for use by physicians. The presence of calcium is correlated with the degree of plaque burden. Detrano et al.16) compared participants with scores above 100 and 300 with participants with no coronary calcium, and found increased coronary events in the higher scoring groups.12) Similar to their study, we also consider that a CACS of above 100 is associated with high risk.
The relationships between the CACS and classical cardiovascular risk factors such as patient age, DM, and HT are well known.17)18) Atherosclerosis is a disease of aging,19) specifically, a state of premature vascular aging that has been related to reduced cell proliferation, irreversible growth arrest and apoptosis, elevated DNA damage, epigenetic modifications, cellular senescence, and telomere shortening.20) Our results support the idea that aging is directly responsible for the development of atherosclerosis. According to a review of the literature by Sunkara et al.18) use of the CACS is a very useful risk stratification technique, primarily for diabetics and patients with metabolic syndrome. Because the CACS is a predictor of atherosclerosis, in our study, as expected, the FRS was found to be elevated in patients with a higher CACS.
As part of an extensively used basic haematological test, the RDW has become a target of investigation after it was found to be a surprisingly strong independent predictor of morbidity and mortality rate in heart failure patients.3) Karabulut et al.5) reported that an elevated RDW level on admission is associated with poorer reperfusion in patients with AMI treated with a primary coronary intervention. Lappe et al.2) found an increase in patient mortality rates with increased RDW levels in their study, including CHD patients who do not have heart failure. Stroke occurrence, CV-related and all-cause mortality were also found to be related to an elevated RDW.21) Another recent study documented that an increased baseline RDW level and an accelerated increase of the RDW in patients with transcatheter aortic valve implantation were associated with a poorer prognosis and increased mortality rate during a median follow-up period of 12 months.6)
Afonso et al.7) emphasized that the RDW is found to be elevated in patients with microalbuminuria. Our earlier study also included patients with another subclinical state of CVD who are free of obvious CHD but who have a low to intermediate risk of developing CHD in the next 10 years.11) We demonstrated a significant relationship between the RDW and the CACS. There are conflicting data in the literature regarding this issue. In contrast to our study, Chaikriangkrai et al.10) found no association between the complete blood cell count, including the RDW, and the CACS in 868 patients without known CHD.
Some studies have linked an increased RDW with inflammatory markers such as high-sensitivity C-reactive protein, soluble tumour necrosis factor receptors, and IL-6 in the setting of atherosclerosis.22)23)24) There is a relationship between inflammation and oxidative stress through activation of nuclear factor-κB via reactive oxygen species.15)25)26) Recently it was shown that recurrent haemorrhages originating from fragile neomicrovessels bleed into the core of unstable plaques.27) This could cause a decline in haemoglobin levels, although this has not yet been demonstrated in the literature. In the present study, however, the haemoglobin level was increased, not decreased, in the higher CACS group. This may be because our patient population, which did not have obvious CHD, probably had more stable plaques. In contrast to our study, Chaikriangkrai et al.10) demonstrated that haemoglobin levels, like all the other haematological parameters, were not related to the CACS.
The NLR is simple to calculate, inexpensive, and is emerging as a potential new biomarker of inflammation related to various inflammatory and neoplastic diseases.19) It could also be used to identify individuals at risk for future CVD events.20) Park et al.28) demonstrated that a higher NLR is associated with the CACS and arterial stiffness. An increased neutrophil and/or a decreased lymphocyte count may contribute to the increased NLR in atherosclerosis.29)30) In the current study, the neutrophil count was higher in patients with a CACS >100, without a significant difference in the lymphocyte count, thus resulting in a higher NLR value in patients with a higher CACS.
In conclusion, the results of the present study show that an elevated RDW is associated with a higher CACS, which reflects the atherosclerotic burden in the coronary arteries. A high NLR correlated with a high CACS, as assessed by coronary CT angiography in patients who had a low to intermediate risk for CHD; and the RDW and patient age were found to be independent predictors of the CACS. Additional studies are needed to clarify the predictive value of RDW and NLR in subclinical CHD patients.
Figures and Tables
 | Fig. 1Comparison of red blood cell distribution width between Group I (CACS≤100) and Group II (CACS>100) patients. CACS: coronary artery calcium score. |
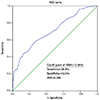 | Fig. 3Receiver-operating characteristic (ROC) curve showed that the cutoff value of red blood cell distribution width=13.05% was an indicator of coronary artery calcification. |
Table 1
Demographic, clinical, and laboratory data for Group I and Group II patients

*Data were given as mean±standard deviation for parametric values and as median (IR) for nonparametric values. CACS: coronary artery calcification score, SD: standard deviation, IR: interquartile range, LVEF: left ventricular ejection fraction, RDW: red blood cell distribution width, WBC: white blood cell, NLR: neutrophil/lymphocyte ratio, LDL: low density lipoprotein, HDL: high density lipoprotein
References
1. Demir A, Yarali N, Fisgin T, Duru F, Kara A. Most reliable indices in differentiation between thalassemia trait and iron deficiency anemia. Pediatr Int. 2002; 44:612–616.
2. Lappé JM, Horne BD, Shah SH, et al. Red cell distribution width, C-reactive protein, the complete blood count, and mortality in patients with coronary disease and a normal comparison population. Clin Chim Acta. 2011; 412:2094–2099.
3. Felker GM, Allen LA, Pocock SJ, et al. Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM Program and the Duke Databank. J Am Coll Cardiol. 2007; 50:40–47.
4. Ye Z, Smith C, Kullo IJ. Usefulness of red cell distribution width to predict mortality in patients with peripheral artery disease. Am J Cardiol. 2011; 107:1241–1245.
5. Karabulut A, Uyarel H, Uzunlar B, Çakmak M. Elevated red cell distribution width level predicts worse postinterventional thrombolysis in myocardial infarction flow reflecting abnormal reperfusion in acute myocardial infarction treated with a primary coronary intervention. Coron Artery Dis. 2012; 23:68–72.
6. Aung N, Dworakowski R, Byrne J, et al. Progressive rise in red cell distribution width is associated with poor outcome after transcatheter aortic valve implantation. Heart. 2013; 06. 04. [Epub]. DOI: 10.1136/heartjnl-2013-303910.
7. Afonso L, Zalawadiya SK, Veeranna V, Panaich SS, Niraj A, Jacob S. Relationship between red cell distribution width and microalbuminuria: a population-based study of multiethnic representative US adults. Nephron Clin Pract. 2011; 119:c277–c282.
8. Wexler L, Brundage B, Crouse J, et al. Coronary artery calcification: pathophysiology, epidemiology, imaging methods, and clinical implications. A statement for health professionals from the American Heart Association. Writing Group. Circulation. 1996; 94:1175–1192.
9. Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol. 2005; 46:158–165.
10. Chaikriangkrai K, Kassi M, Alchalabi S, et al. Association between hematological indices and coronary calcification in symptomatic patients without history of coronary artery disease. N Am J Med Sci. 2014; 6:433–439.
11. Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998; 97:1837–1847.
12. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990; 15:827–832.
13. Margolis JR, Chen JT, Kong Y, Peter RH, Behar VS, Kisslo JA. The diagnostic and prognostic significance of coronary artery calcification. A report of 800 cases. Radiology. 1980; 137:609–616.
14. Budoff MJ, Shaw LJ, Liu ST, et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007; 49:1860–1870.
15. Kondos GT, Hoff JA, Sevrukov A, et al. Electron-beam tomography coronary artery calcium and cardiac events: a 37-month follow-up of 5635 initially asymptomatic low- to intermediate-risk adults. Circulation. 2003; 107:2571–2576.
16. Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008; 358:1336–1345.
17. Olson JC, Edmundowicz D, Becker DJ, Kuller LH, Orchard TJ. Coronary calcium in adults with type 1 diabetes: a stronger correlate of clinical coronary artery disease in men than in women. Diabetes. 2000; 49:1571–1578.
18. Sunkara N, Wong ND, Malik S. Role of coronary artery calcium in cardiovascular risk assessment. Expert Rev Cardiovasc Ther. 2014; 12:87–94.
19. Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013; 88:218–230.
20. Bhat T, Teli S, Rijal J, et al. Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expert Rev Cardiovasc Ther. 2013; 11:55–59.
21. Ani C, Ovbiagele B. Elevated red blood cell distribution width predicts mortality in persons with known stroke. J Neurol Sci. 2009; 277:103–108.
22. Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005; 352:1011–1023.
23. Lippi G, Targher G, Montagnana M, Salvagno GL, Zoppini G, Guidi GC. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med. 2009; 133:628–632.
24. de Gonzalo-Calvo D, de Luxán-Delgado B, Rodríguez-González S, et al. Interleukin 6, soluble tumor necrosis factor receptor I and red blood cell distribution width as biological markers of functional dependence in an elderly population: a translational approach. Cytokine. 2012; 58:193–198.
25. Lippi G, Dipalo M, Teti L, Cervellin G. Relationship between red blood cell distribution width and prognostic biomarkers in patients admitted to the emergency department with acute infections. Eur J Intern Med. 2013; 24:e15–e16.
26. Wang T, Zhang X, Li JJ. The role of NF-kappaB in the regulation of cell stress responses. Int Immunopharmacol. 2002; 2:1509–1520.
27. Chistiakov DA, Orekhov AN, Bobryshev YV. Contribution of neovascularization and intraplaque haemorrhage to atherosclerotic plaque progression and instability. Acta Physiol (Oxf). 2015; 213:539–553.
28. Park BJ, Shim JY, Lee HR, et al. Relationship of neutrophil-lymphocyte ratio with arterial stiffness and coronary calcium score. Clin Chim Acta. 2011; 412:925–929.
29. Paulsson J, Dadfar E, Held C, Jacobson SH, Lundahl J. Activation of peripheral and in vivo transmigrated neutrophils in patients with stable coronary artery disease. Atherosclerosis. 2007; 192:328–334.
30. Núñez J, Miñana G, Bodi V, et al. Low lymphocyte count and cardiovascular diseases. Curr Med Chem. 2011; 18:3226–3233.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download