Abstract
Implantable cardioverter-defibrillator (ICD) therapy is acknowledged as a valid treatment method for the effective prevention of sudden cardiac death, which is a major cause of mortality in adult congenital heart disease patients. But ICD implantation by the conventional transvascular approach is not always possible in patients who have undergone palliative surgery due to congenital and structural heart disease. Here, we report a case in which an ICD was transvascularly implanted in an arrhythmogenic right ventricular cardiomyopathy patient who had undergone a one-and-a-half ventricle repair.
Implantable cardioverter-defibrillator (ICD) therapy is acknowledged as a valid treatment method for the effective prevention of sudden cardiac death.1) Sudden cardiac death is a major cause of mortality (19-26%) in adult congenital heart disease (ACHD) patients.2) Since the survival rate of patients with congenital heart disease has risen due to improved surgical methods and peri-operative management, the number of ACHD patients has also increased recently. Consequently, the importance of the proper management of long term complications in these patients has garnered attention. Therefore, the need of ICD therapy in ACHD patients is gaining momentum.3) We report a case in which an ICD was transvascularly implanted in a patient who had undergone a one-and-a-half ventricle repair.
A 31-year-old male patient presented to the emergency department with symptoms including severe palpitation and a presyncopal episode. His blood pressure was 80/60 mm Hg and the electrocardiogram showed monomorphic ventricular tachycardia (VT), at a rate of 225 bpm (Fig. 1). In the emergency department, cardioversion was performed and the tachycardia was converted into a sinus rhythm.
The patient was diagnosed with right heart failure at the age of 10, when he visited our clinic due to dyspnea on exertion and generalized edema. The patient was followed up in the outpatient clinic regularly with heart failure medications. However, right ventricular (RV) dysfunction occurred and RV enlargement progressed. Because the severe RV dysfunction was unresponsive to medical treatment, the patient underwent a one-and-a-half ventricle repair along with tricuspid annuloplasty and valvuloplasty. One-and-a-half ventricle repair is an operation to create an end-to-side anastomosis of the superior vena cava (SVC) and the right pulmonary artery (a bidirectional cavopulmonary shunt) in conjunction with standard heart repair. A partial diversion of systemic venous return directly to the pulmonary artery reduces the volume load on the right ventricle. The schematic picture for describing the one-and-a-half ventricle repair was drawn on the patient's chest X-ray (Fig. 2A). After the one-and-a-half ventricle repair, the patient's symptoms improved dramatically and, against our advice, he stopped visiting the clinic.
The echocardiogram performed at the age of 31 still showed severe RV enlargement with RV dyskinesia. Tricuspid valve regurgitation was improved compared with that before the operation. The connection between the SVC and the pulmonary artery created during the surgical procedure was intact. To better assess RV function, a heart magnetic resonance image (MRI) was performed. The heart MRI showed marked RV enlargement, dyskiensia, wall thinning, and extensive delayed hyperenhancement, indicative of fibrous displacement (Fig. 3). These MRI findings met the criteria for a diagnosis of arrhythmogenic RV cardiomyopathy (ARVC). The patient had a hemodynamically unstable VT due to ARVC, which was caused by the severe RV dysplasia and dysfunction, and the decision to insert the single-chamber ICD was made. The ICD could not be transvenously implanted in the conventional manner, because the SVC was connected to the pulmonary artery. We decided to use the transvascular approach through the right axillary vein to implant the ICD. We first threaded a catheter through the femoral vein and performed a right subclavian vein angiogram to confirm an intact flow through the connection between the SVC and the pulmonary artery. The right axillary vein was punctured. A long wire was smoothly passed through subclavian vein, SVC, cavopulmonary shunt, pulmonary artery, and pulmonic valve sequentially, ultimately reaching the RV. A long peel-away sheath was introduced following the long wire and a screw type ICD lead (DURATA 7120Q 65 cm, St. Jude Medical, Valley View Court Sylmar, CA, USA) was inserted within the RV. The ICD lead was first placed at the RV apex, as in the conventional manner, and was stably anchored. However, the electrophysiologic properties were not adequate, and thus no signal was captured. Hence, the ICD lead was repositioned at the anteroseptal junction, where the RV electrical signal was satisfactory. The ICD lead was stably anchored at that position. The ICD generator (Ellipse VR, St. Jude Medical, Valley View Court Sylmar, CA, USA) was placed at the right pectoral area and we sutured the wound and finished the procedure. After the procedure, we performed an ICD interrogation and defibrillation threshold test. The defibrillation threshold was 10 joules, and the R wave amplitude, pacing threshold and impedance were 7.0 mV, 0.6 V at 0.5 ms, and 550 ohms, respectively. On the last outpatient clinic follow-up, the implanted ICD lead was in a stable position (Fig. 2B) and normal pulmonic valve function was noted.
Up until now, ICD insertion was not possible by the conventional transvascular approach in patients diagnosed with congenital and structural heart disease that underwent palliative surgery resulting in hemodynamically normal heart.4)5) In these patients, ICD implantation can be performed occasionally, and recently, cases involving groups or a single patient have been reported.6)7) Also, subcutaneous ICDs can be used as an alternative method in cases in which a normal vascular approach is not feasible.8)9) However, the subcutaneous ICD is not yet a commercially available treatment option in Korea. One-and-a-half ventricle repair is an effective palliative surgical method which reduces the RV volume in congenital heart disease patients with RV hypoplasia or dysfunction.10) In patients who undergo a one-and-a-half ventricle repair, fatal arrhythmias requiring ICD implantation could occur due to the underlying congenital heart disease. But, the ICD cannot be transvenously implanted in the conventional manner because the SVC is connected to the pulmonary artery in these patients. So, an epicardial approach has been used. The epicardial approach is an invasive procedure in comparison to the transvascular approach. In our study, we inserted an ICD in the right pectoral area using the transvascular approach through the right axillary vein and bidirectional cavopulmonary shunt in a patient who had undergone a one-and-a-half ventricle repair. To our knowledge, this is the creative method that has not been reported before.
In conclusion, we can feasibly perform single-chamber ICD implantation in a patient who has undergone one-and-a-half ventricle repair through the conventional vascular approach instead of the epicardial approach. This can be accepted as an effective treatment option.
Figures and Tables
Fig. 1
Patient's ECG when he visited the emergency department with palpitations and pre-syncope. ECG showed monomorphic ventricular tachycardia at the rate of 225 bpm. ECG: electrocardiogram.

Fig. 2
A schematic picture describing the one-and-a-half ventricle repair and chest X-ray image of the implanted ICD. A: a schematic picture describing the one-and-a-half ventricle repair is drawn on the patient's chest X-ray. Gray arrows represent the blood flow. B: the chest X-ray shows that the ICD is placed in the right pectoral area in the RV via the cavopulmonary shunt. ICD: implantable cardioverter-defibrillator, PA: pulmonary artery, RV: right ventricle, SVC: superior vena cava.
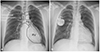
Fig. 3
Heart magnetic resonance images. Short axis cine image with SSFP sequence (A), four chamber cine image with SSFP sequence (B), delayed enhanced image (C). Severe right ventricular enlargement with a dyskinetic segment and right ventricular wall thinning is noted (arrowheads). In the contrast study, extensive delayed hyperenhancement which indicates fi brous displacement is also shown (arrow). These fi ndings of the heart MRI meet the criteria for a diagnosis of ARVC. ARVC: arrhythmogenic right ventricular cardiomyopathy, MRI: magnetic resonance imaging, SSFP: steady state free precession.

References
1. Winkle RA, Mead RH, Ruder MA, et al. Long-term outcome with the automatic implantable cardioverter-defibrillator. J Am Coll Cardiol. 1989; 13:1353–1361.
2. Koyak Z, Harris L, de Groot JR, et al. Sudden cardiac death in adult congenital heart disease. Circulation. 2012; 126:1944–1954.
3. Mondésert B, Khairy P. Implantable cardioverter-defibrillators in congenital heart disease. Curr Opin Cardiol. 2014; 29:45–52.
4. Cannon BC, Friedman RA, Fenrich AL, Fraser CD, McKenzie ED, Kertesz NJ. Innovative techniques for placement of implantable cardioverter-defibrillator leads in patients with limited venous access to the heart. Pacing Clin Electrophysiol. 2006; 29:181–187.
5. Uyeda T, Inoue K, Sato J, et al. Outcome of implantable cardioverter defibrillator therapy for congenital heart disease. Pediatr Int. 2012; 54:379–382.
6. Nery PB, Green MS, Khairy P, Alhebaishi Y, Hendry P, Birnie DH. Implantable cardioverter-defibrillator insertion in congenital heart disease without transvenous access to the heart. Can J Cardiol. 2013; 29:254.
7. Khanna AD, Warnes CA, Phillips SD, Lin G, Brady PA. Single-center experience with implantable cardioverter-defibrillators in adults with complex congenital heart disease. Am J Cardiol. 2011; 108:729–734.
8. Akerström F, Arias MA, Pachón M, Puchol A, Jiménez-López J. Subcutaneous implantable defibrillator: state-of-the art 2013. World J Cardiol. 2013; 5:347–354.
9. Lambiase PD, Barr C, Theuns DA, et al. Worldwide experience with a totally subcutaneous implantable defibrillator: early results from the EFFORTLESS S-ICD Registry. Eur Heart J. 2014; 35:1657–1665.
10. Kreutzer C, Mayorquim RC, Kreutzer GO, et al. Experience with one and a half ventricle repair. J Thorac Cardiovasc Surg. 1999; 117:662–668.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download