Abstract
Primary aortoenteric fistula is a direct communication between the aorta and intestinal lumen and it represents a rare but potentially lethal complication of an abdominal aortic aneurysm. However, it may occur less frequently in a naive non-aneurysmatic aorta. Diagnosis is often difficult and delayed in most cases, unless there is a high level of clinical awareness. Urgent surgery is still the recommended treatment. We describe the case of primary aortoenteric fistula of a saccular aneurysm. A 55-year-old woman was referred to our center with hematemesis, melena, and severe anemia who was dignosed previously with unknown saccular abdominal aneurysm.
Primary aortoenteric fistula is a rare but potentially lethal complication of an abdominal aortic aneurysm. Diagnosis is often difficult and very challenging. We report the case of a woman with an acute episode of hematemesis.
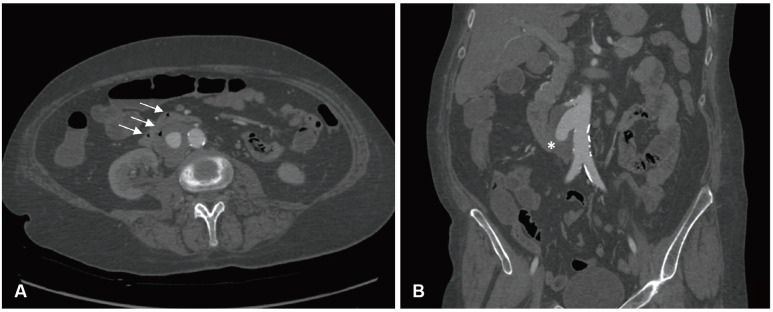
A 55-year-old woman was referred to the emergency room for syncope associated with hematemesis, melena and anemia. The patient's history revealed a heavy smoking habit, chronic alcohol-related liver disease and congenital hiatal hernia. She reported a similar episode of hematemesis five days prior but at that time refused further investigation. On arrival in the emergency room, she was hemodynamically stable, orientated and alert. A gastro-intestinal endoscopy was performed to better understand the possible cause of bleeding. The exam revealed many coagulated blood spots in the stomach and a clot tenaciously adhered to the duodenal wall without active bleeding signs or esophageal varices. A computed tomography (CT) scan showed a saccular abdominal aortic aneurysm (max diameter: 44 mm), a few centimeters downstream from the origin of the right renal artery, the presence of multiple gas bubbles in the thrombus, and the presence of a strong adherence between the aneurysm and the third portion of the duodenum (Fig. 1). The patient was unaware of this pathology. According to these clinical signs and images she was urgently taken to the operating room with the diagnosis of primary aorto-enteric fistula (PAEF). A team of vascular and general surgeons performed the procedure. The saccular aneurysm and the duodenum were isolated by a transperitoneal approach after accurate debridement of some adherences between the two structures. We decided to clamp the aorta above the right renal artery because of the proximity of the aneurysm and the origin of the vessel. We used a Dacron Silver graft to perform an aorto-aortic substitution sutured with a synthetic nonabsorbabable monofilament Prolene. Proximal clamping lasted 12 minutes and during that time the right kidney perfusion was guaranteed by the infusion of crystalloid fluids at 5℃ at a flow rate of 1.5 mL/g/min (CUSTODIOL® Benshein, Germany). Due to a limited extension of the duodenal tear, the intestinal wall was directly sutured and a portion of omentum was interposed between the graft and the bowel (Fig. 2). The patient required the transfusion of four units of packed red blood cells. An intra-operative bacterial culture obtained from the aortic wall was negative. As a result, a broad-spectrum antibiotic prophylaxis with metronidazol, teicoplanin and fluconazol was administered. Parenteral feeding therapy was set up for 12 days. A CT scan and Gastrografin (Bracco Diagnostic Inc., Quebec, Canada) intestinal transit tests were finally performed showing the success of the repair. On the sixteenth day after the surgery, the woman was discharged in a good clinical condition.
Primary aorto-enteric fistula is defined as a direct communication between the aorta and the gastro-intestinal lumen and represents a rare and severe complication of the abdominal aortic aneurysm (AAA). The primary form, less common with an incidence of 0.04 to 0.07%, is due to the erosion of the intestinal wall by an aortic aneurysm.1)2) Approximately 350 cases of PAEF have been reported in the literature since its first description about 200 years ago.3) On the other hand, secondary aorto-enteric fistula has an incidence of 0.4-1.6% and may represent the complication of surgical aortic replacement.2) Mortality is extremely high: around 100% of untreated patients or patients with active bleeding, and around 30-40% of patients treated with surgery4) Epidemiological data reveal that the average age of diagnosed PAEF is 64-years-old and that it mainly affects males (3:1 ratio).5) About 83% of PAEF is associated with a fusiform AAA (mean diameter 6.2 cm).6)7) Other causes of PAEF are related to infectious diseases such as syphilis, tuberculosis, mycotic infections, and collagen diseases.8) Moreover, gastric pathologies (peptic ulcer, intestinal tumors, pancreatitis), history of radiation exposure and neoplastic diseases appear to increase the risk of PAEF.8)9) The intestinal tracts more frequently involved in PAEF are the second and third portion of the duodenum and the duodeno-jejunal angle (54%).7) The clinical presentation of PAEF is often insidious unless doctors suspect gastro-intestinal bleeding (80%)10) with episodes of hematemesis and melena (64%), abdominal pain (32%) and the palpation of an abdominal pulsating mass (25%).11) The onset of PAEF is rarely related to signs of sepsis (fever, leukocytosis) or with hemorrhagic shock with hypotension, tachycardia, and loss of conciousness.12) The triad of abdominal pain, pulsatile mass and gastrointestinal bleeding is described in only 11% of patients.6) Despite the absence of a pathognomonic sign, there is a characteristic presentation of PAEF defined as "herald bleeding"13)14) It consists of an episode of gastro-intestinal bleeding which is usually self-limiting by formation of a blood clot in the aorto-enteric fistula. However, after infusion therapy, increasing blood pressure or endoscopic procedures, the clot can be removed leading to severe bleeding, with a tendency of hemodynamic instability.2) Gastrointestinal endoscopy is the first-step in detecting gastrointestinal bleeding of unknown origin but the sensitivity for detecting PAEF is < 25%.6) Many authors agree that this examination requires experienced operators because of the necessity to evaluate the duodenum in its entire length and to avoid reckless maneuvers leading to the removal of the clot.7) The diagnosis of PAEF is usually obtained through a CT scan showing air bubbles in the aortic wall and the presence of contrast medium in the intestinal lumen. The sensitivity of this technique varies from 30 to 61%.15) The gold standard in the treatment of PAEF is surgery. In our case study, we performed an aorto-aortic bypass with a Dacron Silver prosthesis, which is the preferred choice in a septic or potentially septic area. The advantages of this method are the relative speed of execution, the good long-term patency, and the preservation of the anatomic course of the vessels. Other treatment options proposed in the literature include extra-anatomic by-pass, the substitution of the aortic tract with a venous prosthesis (neoaortoiliac system, NAIS) and the cryopreserved arterial homografts.15)16)17) The endovascular aneurysm repair (EVAR) of the PAEF, as reported in the literature, could be applied in specific situations as a "bridge" treatment in patients with haemodynamic instability, where immediate surgery is contra-indicated and has to be delayed.18)19) This solution is still controversial, especially for the persistence of the infective site and the risk of EVAR's infection.20)
In conclusion, a saccular aneurysm, too small to require elective surgical treatment, is a possible cause of early onset PAEF. It is mandatory to consider PAEF in differential diagnoses even in patients without a history of aneurysm but with other risk factors, such as smoking and gastro-intestinal inflammatory diseases, in order to provide prompt surgical treatment.
References
1. Lemos DW, Raffetto JD, Moore TC, Menzoian JO. Primary aortoduodenal fistula: a case report and review of the literature. J Vasc Surg. 2003; 37:686–689. PMID: 12618713.
2. Chenu C, Marcheix B, Barcelo C, Rousseau H. Aorto-enteric fistula after endovascular abdominal aortic aneurysm repair: case report and review. Eur J Vasc Endovasc Surg. 2009; 37:401–406. PMID: 19211278.
3. Philippakis GE, Moustardas M. Surgical treatment of primary aortojejunal fistula. Int J Surg Case Rep. 2013; 4:477–479. PMID: 23557938.
4. Genovés-Gascó B, Torres-Blanco Á, Plaza-Martínez Á, Olmos-Sánchez D, Gómez-Palonés F, Ortiz-Monzón E. Primary aortoduodenal fistula in a patient with pararenal abdominal aortic aneurysm. Ann Vasc Surg. 2012; 26:730.e1–730.e5. PMID: 22503432.
5. Saers SJ, Scheltinga MR. Primary aortoenteric fistula. Br J Surg. 2005; 92:143–152. PMID: 15685700.
6. Ranasinghe W, Loa J, Allaf N, Lewis K, Sebastian MG. Primary aorto-enteric fistulae: the challenges in diagnosis and review of treatment. Ann Vasc Surg. 2011; 25:386.e1–386.e5. PMID: 21269801.
7. Senadhi V, Brown JC, Arora D, Shaffer R, Shetty D, Mackrell P. A mysterious cause of gastrointestinal bleeding disguising itself as diverticu-losis and peptic ulcer disease: a review of diagnostic modalities for aortoenteric fistula. Case Rep Gastroenterol. 2010; 4:510–517. PMID: 21151635.
8. Song Y, Liu Q, Shen H, Jia X, Zhang H, Qiao L. Diagnosis and management of primary aortoenteric fistulas--experience learned from eighteen patients. Surgery. 2008; 143:43–50. PMID: 18154932.
9. Shulik O, Marling K, Butler J. Primary aorto-enteric fistula - a unique complication of poorly differentiated large B-cell lymphoma. Am J Case Rep. 2013; 14:194–197. PMID: 23826466.
10. Kim JS, Han JH, Kang MH, et al. [A case of primary aortoenteric fistula mimicking ulcer bleeding]. Korean J Gastroenterol. 2013; 61:343–346. PMID: 23877216.
11. Lee CW, Chung SW, Song S, Bae MJ, Huh U, Kim JH. Double primary aortoenteric fistulae: a case report of two simultaneous primary aorto-enteric fistulae in one patient. Korean J Thorac Cardiovasc Surg. 2012; 45:330–333. PMID: 23130309.
12. Ramanujam S, Shiels A, Zuckerman G, Prakash C. Unusual presentations of aorto-enteric fistula. Gastrointest Endosc. 2004; 59:300–304. PMID: 14745412.
13. Lawlor DK, DeRose G, Harris KA, Forbes TL. Primary aorto/iliac-enteric fistula-report of 6 new cases. Vasc Endovascular Surg. 2004; 38:281–286. PMID: 15181513.
14. Bala M, Sosna J, Appelbaum L, Israeli E, Rivkind Al. Enigma of primary aortoduodenal fistula. World J Gastroenterol. 2009; 15:3191–3193. PMID: 19575502.
15. Berger P, Moll FL. Aortic graft infections: is there still a role for axillo-bifemoral reconstruction? Semin Vasc Surg. 2011; 24:205–210. PMID: 22230675.
16. Bisdas T, Bredt M, Pichlmaier M, et al. Eight-year experience with cryopreserved arterial homografts for the in situ reconstruction of abdominal aortic infections. J Vasc Surg. 2010; 52:323–330. PMID: 20570473.
17. Chung J, Clagett GP. Neoaortoiliac System (NAIS) procedure for the treatment of the infected aortic graft. Semin Vasc Surg. 2011; 24:220–226. PMID: 22230677.
18. Setacci C, de Donato G, Setacci F. Endografts for the treatment of aortic infection. Semin Vasc Surg. 2011; 24:242–249. PMID: 22230680.
19. Malas MB, Saha S, Qazi U, et al. Is endovascular stent-graft treatment of primary aortoesophageal fistula worthwhile? Vasc Endovascular Surg. 2011; 45:83–89. PMID: 20810403.
20. Danneels MI, Verhagen HJ, Teijink JA, Cuypers P, Nevelsteen A, Vermassen FE. Endovascular repair for aorto-enteric fistula: a bridge too far or a bridge to surgery? Eur J Vasc Endovasc Surg. 2006; 32:27–33. PMID: 16427330.
Fig. 1
Pre-operative CT scan. A: transverse section of the arterial phase CT scan of the abdominal saccular aneurysm with the presence of gas bubbles in the intestinal lumen (white arrows). B: sagittal section of the arterial phase CT scan of the abdominal aorta highlighting the saccular morphology of the aneurysm and the close relationship between the duodenum (*) and the vessel wall. CT: computed tomography.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download