Abstract
Rupture of a vulnerable plaque and subsequent thrombus formation are important mechanisms leading to the development of an acute myocardial infarction (AMI). Typical intravascular ultrasound (IVUS) features of AMI include plaque rupture, thrombus, positive remodeling, attenuated plaque, spotty calcification, and thin-cap fibroatheroma. No-reflow phenomenon was attributable to the embolization of thrombus and plaque debris that results from mechanical fragmentation of the vulnerable plaque by percutaneous coronary intervention (PCI). Several grayscale IVUS features including plaque rupture, thrombus, positive remodeling, greater plaque burden, decreased post-PCI plaque volume, and tissue prolapse, and virtual histology-IVUS features such as large necrotic corecontaining lesion and thin-cap fibroatheroma were the independent predictors of no-reflow phenomenon in AMI patients. Non-culprit lesions associated with recurrent events were more likely than those not associated with recurrent events to be characterized by a plaque burden of ≥70%, a minimal luminal area of ≤4.0 mm2, or to be classified as thin-cap fibroatheromas.
Acute myocardial infarction (AMI) results from spontaneous plaque rupture or erosion and subsequent thrombosis.1)2) Intravascular ultrasound (IVUS) has a pivotal role in detecting plaque characteristics and periprocedural complications, and assessing plaque evolution after medical therapy. Pre-percutaneous coronary intervention (PCI) provides measures of vessel sizes, lumen, plaque and plaque length; assesses plaque morphology, calcium, and remodeling pattern; detects vulnerable plaque including plaque rupture, thrombus, attenuated plaque, and thin-cap fibroatheroma (TCFA); detects dissection and aneurysm; and determines stent size and length.3) IVUS can predict post-PCI distal embolization or no-reflow. Many IVUS predictors of peri-procedural myocardial infarction (MI) have been demonstrated, including thrombus formation,4)5) greater plaque burden,4)5) positive remodeling,6)7) and decreased post-PCI plaque volume,5)8) and TCFA.9) IVUS is very useful to assess plaque changes and its impact on long-term clinical outcomes.10)11)12) Recently, Singh et al.13) reported that IVUS guidance in patients with AMI is associated with reduced in-hospital mortality, similar length of hospital stay, and increased cost of care and vascular complications, as compared with conventional angiography-guided PCIs.
The authors have reviewed pre- and post-PCI IVUS findings and predictors of peri-procedural MI and plaque progression/regression in patients with AMI.
Rupture of vulnerable plaque and/or endothelial erosions with subsequent thrombus formation are the main mechanisms implicated in the pathogenesis of AMI. Fig. 1A showed a ruptured plaque containing a cavity that communicated with the lumen with an overlying residual fibrous cap fragment.14) Rupture sites separated by a length of artery containing smooth lumen contours without cavities represent multiple plaque ruptures. In the our previous study,15) ruptured plaque was observed in 46% patients with ST-segment elevation myocardial infarction (STEMI) and 29% patients with non-ST-segment elevation myocardial infarction (NSTEMI) (p=0.002); furthermore, multiple plaque ruptures tended to be more frequent in patients with STEMI, as compared with those with NSTEMI (19% vs. 13%, p=0.14). However, plaque cavity cross-sectional area (CSA) and ruptured plaque length were not different between patients with STEMI and those with NSTEMI (2.34±1.16 mm2 vs. 2.33±1.58 mm2, p=0.9, and 2.69±1.11 mm vs. 2.67±1.67 mm, p=0.9, respectively). Kusama et al.16) reported that plaque rupture was associated with morphologic characteristics of vulnerable lesions with a higher incidence of soft plaque and positive remodeling. Plaque rupture is closely related to obstructive thrombus formation and the longitudinal morphology of plaque rupture also affects the coronary flow. The presence of thrombi may obscure IVUS detection of plaque rupture.
The identification of thrombus requires at least 2 of the following: distinct hypoechoic mass, brightly speckled plaque, channeling within the plaque, evacuated plaque cavity, or detached mobile mass (Fig. 1B).17) The detection rate of thrombus by IVUS is not high due to the limited resolution of IVUS. Injection of contrast or saline may disperse the stagnant flow, clear the lumen, and allow differentiation of stasis from thrombosis. However, none of these features is pathognomic for thrombus, and the diagnosis of thrombus by IVUS should always be considered presumptive.3) In our previous study,15) IVUS-detected thrombus was observed in 34% patients with STEMI and 21% patients with NSTEMI (p=0.006).
Coronary artery remodeling is assessed by comparing the lesion site to the reference external elastic membrane (EEM) CSA. Remodeling index is the lesion site EEM CSA divided by the average of the proximal and distal reference EEM CSA. Positive remodeling is defined as a remodeling index >1.05 (Fig. 1C), intermediate remodeling as a remodeling index between 0.95 and 1.05, and negative remodeling as a remodeling index <0.95.18) In patients with AMI, Hasegawa et al.19) reported that 55% cases showed positive remodeling on IVUS, whereas negative remodeling was observed in 25% cases; in addition, patients with positive remodeling were significantly older than those with negative remodeling, and the frequency of calcifications was higher and soft plaque with small calcifications was the more frequent in patients with positive remodeling than those with negative remodeling.
Attenuated plaque is defined as hypoechoic plaque with deep ultrasound attenuation without calcification or very dense fibrous plaque (Fig. 1D).20) Wu et al.21) reported that 78% of the AMI patients had attenuated plaques in the Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial. Lee et al.22) reported that attenuated plaque was observed in 39.6% of STEMI and 17.6% of NSTEMI (p<0.001). Furthermore, the level of C-reactive protein (CRP) was higher and angiographic thrombus and initial Thrombolysis In Myocardial Infarction (TIMI) flow grade <2 were more common; IVUS lesion site plaque burden and remodeling index were significantly greater; lesion site luminal dimensions were significantly smaller; and thrombus, positive remodeling, and plaque rupture were more common in AMI patients with attenuated plaque, as compared with those without attenuated plaque.
Plaque composition plays a role in plaque disruption and thrombosis that leads to acute coronary events.23) Lesions with a large lipid core have a higher risk for disruption than sclerotic plaques.24) TCFA by virtual histology (VH)-IVUS is defined as a necrotic core (NC) ≥10% of plaque area in at least 3 consecutive frames without overlying fibrous tissue in the presence of ≥40% plaque burden (Fig. 1E).25) TCFA is the precursor of plaque rupture that accounts for a majority of coronary thrombi and coronary death.22) Previous study has shown that VH-IVUS identified TCFA is a more prevalent finding in patients with AMI than in stable angina patients.26)
Cardiovascular disease is highly prevalent among the elderly who constitute an increasing segment of the population, accounting for most of their morbidity and mortality. Compared with the general population, elderly patients undergoing coronary revascularization are more likely to present with more complex lesions, unstable angina, comorbid conditions and a lower ejection fraction.27)28) Hassani et al.29) reported that rupture/dissection were observed more frequently (32% vs. 9%, p=0.009) and culprit lesions contained more thrombus (14% vs. 2%, p=0.04) in the <65-year-old group; conversely, in octogenarians, lesions were predominantly calcified (57% vs. 10%, p<0.001) and longer (20.9±7.8 mm vs. 16.6±6.1 mm, p=0.004) with less positive remodeling (19% vs. 56%, p<0.001), and age was the only independent predictor of calcified plaque (p=0.02) and remodeling (p=0.005) in AMI patients.
Plaque rupture frequently occurs at the site of thinnest fibrous cap with heavy infiltration of activated macrophage foam cells, indicating ongoing inflammation at the site of plaque disruption. Inflammation plays an important role in both atherogenesis and atherothrombotic events. High-sensitivity CRP is associated with increased risk for coronary artery disease, and myocardial necrosis further promotes the synthesis of CRP. Sano et al.30) reported that plaque rupture was observed more frequently in the elevated CRP group (≥3 mg/L) than in the normal CRP group (70% vs. 43%, p=0.01) and the presence of ruptured plaque alone correlated with elevation of serum CRP {p=0.02; odds ratio (OR), 3.35; 95% confidence interval (CI), 1.22 to 9.18}. Tanaka et al.31) reported that high-sensitivity CRP levels had a positive correlation with the number of plaque ruptures (p<0.01). Therefore, multiple plaque rupture is associated with systemic inflammation, and patients with multiple plaque rupture can be expected to show a poor prognosis.
Tissue prolapse is characterized by an intraluminal tissue extrusion through the stent struts, and is easily detectable using IVUS (Fig. 1F). Several pre-PCI IVUS factors such as soft (rather than fibrous or calcified) plaque, smaller minimal lumen diameter, and larger plaque burden are related to tissue prolapse. The risk of tissue prolapse is known to be higher during aggressive stenting procedures.32)33) In our previous study,34) we assessed the incidence, predictors, and outcome of tissue prolapse after stent implantation in patients with AMI. The tissue prolapse was detected in 27% patients after stent implantation, and stent length, plaque rupture, and positive remodeling were independently associated with the development of tissue prolapse. We also showed that tissue prolapse, plaque rupture, and thrombus were independently associated with post-stenting creatine kinase-myocardial band elevation.
Stent malapposition may be a sign of impaired healing or the result of suboptimal stent implantation. Stent malapposition may increase the thrombotic risk due to the presence of intraluminal stent struts. van der Hoeven et al.35) studied acute and late stent malapposition after implantation of bare-metal stents (BMS) and sirolimus-eluting stents (SES) in STEMI patients who were enrolled in the MISSION! Intervention Study. Acute stent malapposition was found in 38.5% SES patients and 33.8% BMS patients (p=0.51); and late stent malapposition in 37.5% SES patients and 12.5% BMS patients (p<0.001). Acquired stent malapposition was found in 25.0% SES patients and 5.0% BMS patients (p<0.001). Predictors of acute stent malapposition were reference diameter (SES: OR 3.49, 95% CI 1.29 to 9.43; BMS: OR 28.8, 95% CI 4.25 to 94.5) and balloon pressure (BMS: OR 0.74, 95% CI 0.58 to 0.94). Predictors of late stent malapposition were diabetes mellitus (SES: OR 0.16, 95% CI 0.02 to 1.35), reference diameter (BMS: OR 19.2, 95% CI 2.64 to 139.7), and maximum balloon pressure (BMS: OR 0.74, 95% CI 0.55 to 1.00). After SES implantation, acquired stent malapposition was caused by positive remodeling in 84% and plaque reduction in 16% of patients.
The periprocedural MI or no-reflow phenomenon is observed frequently after PCI for AMI. No-reflow phenomenon is attributable to the embolization of thrombus and plaque debris that results from mechanical fragmentation of the vulnerable plaque by PCI. Tanaka et al.6) reported that lipid pool-like image and lesion EEM CSA were independent predictive factors of no-reflow phenomenon after reperfusion for AMI. Kusama et al.36) reported that patients with plaque rupture had a higher incidence of no-reflow phenomenon (15% vs. 3%, p=0.08) and a lower myocardial blush grade (1.5 vs. 2.3, p<0.05) after PCI. Endo et al.20) reported that ultrasound attenuation with a longitudinal length of ≥5 mm and plaque rupture correlated with no-reflow phenomenon in patients with STEMI. Our previous study37) showed that positive remodeling, plaque rupture, IVUS-detected thrombus, and tissue prolapse were independently associated with post-stenting cardiac-specific troponin I elevation. In another previous study,38) we demonstrated that tissue prolapse, high-sensitivity CRP, and culprit lesion multiple plaque ruptures were associated with post-PCI no-reflow in AMI patients with plaque rupture. Ohshima et al.39) reported that cavity volume of ruptured plaque was an independent predictor for angiographic no-reflow phenomenon during primary angioplasty in patients with STEMI. Sato et al.8) reported that the decrease in plaque volume after PCI was significantly larger in patients with inadequate reflow than in those with reflow (49.4±18.9 vs. 31.7±15.5 mm3, p=0.001) and delta-plaque volume was significantly correlated with corrected TIMI frame count after PCI in patients with AMI.
There are controversies in the association between VH-IVUS plaque components and no-reflow or slow-flow phenomenon after PCI in AMI patients.40)41)42)43) In the largest investigation to date regarding relation between plaque composition and no-reflow,9) we demonstrated the NC-rich plaque as assessed by VH-IVUS was independently associated with no-reflow.
Many IVUS predictors for early stent thrombosis (EST) including under expansion of stents, smaller final lumen area and inflow/outflow disease (residual stenosis or dissection) have been reported.44)45)46)47) Previously, we reported that plaque burden at the minimum lumen site was significantly greater, and pre-PCI plaque rupture and IVUS-detected thrombus were observed more frequently, and post-stenting tissue prolapse was observed more frequently in patients with EST, as compared with those without EST (68.8% vs. 32.6%, p=0.003); and tissue prolapse was an independent predictor of EST.48)
Several IVUS studies have demonstrated the benefits of statin therapy in regression or non progression of coronary plaque. Previous IVUS studies have shown that low-density lipoprotein (LDL) cholesterol level was the independent predictor of changes in coronary plaque size.49)50) The Providing Regional Observations to Study Predictors of Events in the Coronary Tree (PROSPECT) trial10) demonstrated that non-culprit lesions associated with recurrent events were more likely than those not associated with recurrent events to be characterized by a plaque burden of ≥70%, a minimal luminal area of ≤4.0 mm2, or to be classified as TCFAs. Lehman et al.51) reported that plaque progression over time was dependent on plaque composition i.e., there was more progression in noncalcified plaque, as compared to calcified plaque. Nicholls et al.52) reported that calcified plaques are more resistant to undergoing changes in size in response to systemic interventions targeting atherosclerotic risk factors. Our previous study,53) indicated that NC is associated with plaque progression in patients when the LDL cholesterol level is around 80 mg/dL at 9 month follow up after treatment with rosuvastatin 10 mg/day. Events related to non-culprit lesions typically occurred at sites that were classified as TCFAs.
IVUS is a useful tool to assess vascular geometry and morphology, and quantitative composition of atherosclerotic plaque at pre-PCI. It is additionally used to assess post-procedural tissue prolapse and malapposition, and to predict post-PCI myonecrosis and EST and plaque progression/regression at long-term follow-up in AMI patients. Although IVUS has limited resolution, it can inform clinical decision making, especially to determine vessel size and arterial remodeling and to select stent size and length, as compared to other imaging modalities such as optical coherence tomography. IVUS guidance can reduce mortality and vascular complications, as compared with conventional angiography-guided PCI in patients with AMI. The recently developed multimodality coronary imaging catheter utilizing combination of IVUS and near-infrared spectroscopy is being used in clinical practice to interrogate the artery and determine the structure and chemistry of the plaque.
Figures and Tables
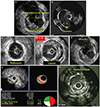
Fig. 1
Intravascular ultrasound findings in patients with acute myocardial infarction. (A) Plaque rupture with a cavity that communicated with the lumen with an overlying residual fibrous cap fragment, (B) intracoronary thrombus shows a distinct hypoechoic mass, (C) positive remodeling with a remodeling index of 1.21, (D) attenuated plaque shows hypoechoic plaque with deep ultrasound attenuation without calcification or very dense fibrous plaque, (E) thin-cap fibroatheroma with a necrotic core of 35.4% of plaque area in the presence of 83.3% of plaque burden, and (F) tissue prolapse shows an intraluminal tissue extrusion through the stent struts.
Acknowledgements
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Korea (HI13C1527), and a grant of the National Research Foundation of Korea Grant funded by the Korean Government (2011-0008875), and a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Korea (HI13C0163), and the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MEST) (2012M3A9C6049744), and Chonnam National University Hospital Research Institute of Clinical Medicine (CRI 11080-21), Korea.
References
1. Davies MJ, Thomas A. Thrombosis and acute coronary-artery lesions in sudden cardiac ischemic death. N Engl J Med. 1984; 310:1137–1140.
2. Farb A, Burke AP, Tang AL, et al. Coronary plaque erosion without rupture into a lipid core. A frequent cause of coronary thrombosis in sudden coronary death. Circulation. 1996; 93:1354–1363.
3. Mintz GS, Nissen SE, Anderson WD, et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001; 37:1478–1492.
4. Iijima R, Shinji H, Ikeda N, et al. Comparison of coronary arterial finding by intravascular ultrasound in patients with "transient no-reflow" versus "reflow" during percutaneous coronary intervention in acute coronary syndrome. Am J Cardiol. 2006; 97:29–33.
5. Katayama T, Kubo N, Takagi Y, et al. Relation of atherothrombosis burden and volume detected by intravascular ultrasound to angiographic no-reflow phenomenon during stent implantation in patients with acute myocardial infarction. Am J Cardiol. 2006; 97:301–304.
6. Tanaka A, Kawarabayashi T, Nishibori Y, et al. No-reflow phenomenon and lesion morphology in patients with acute myocardial infarction. Circulation. 2002; 105:2148–2152.
7. Kotani J, Mintz GS, Castagna MT, et al. Usefulness of preprocedural coronary lesion morphology as assessed by intravascular ultrasound in predicting Thrombolysis In Myocardial Infarction frame count after percutaneous coronary intervention in patients with Q-wave acute myocardial infarction. Am J Cardiol. 2003; 91:870–872.
8. Sato H, Iida H, Tanaka A, et al. The decrease of plaque volume during percutaneous coronary intervention has a negative impact on coronary flow in acute myocardial infarction: a major role of percutaneous coronary intervention-induced embolization. J Am Coll Cardiol. 2004; 44:300–304.
9. Hong YJ, Jeong MH, Choi YH, et al. Impact of plaque components on no-reflow phenomenon after stent deployment in patients with acute coronary syndrome: a virtual histology-intravascular ultrasound analysis. Eur Heart J. 2011; 32:2059–2066.
10. Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011; 364:226–235.
11. Kim WH, Park HW, Kim KH, et al. Fibro-fatty component is important for the long-term clinical events in patients who have undergone primary percutaneous coronary intervention. Korean Circ J. 2012; 42:33–39.
12. Kim KH, Kim WH, Park HW, et al. Impact of plaque composition on long-term clinical outcomes in patients with coronary artery occlusive disease. Korean Circ J. 2013; 43:377–383.
13. Singh V, Badheka AO, Arora S, et al. Comparison of inhospital mortality, length of hospitalization, costs, and vascular complications of percutaneous coronary interventions guided by ultrasound versus angiography. Am J Cardiol. 2015; 115:1357–1366.
14. Maehara A, Mintz GS, Bui AB, et al. Morphologic and angiographic features of coronary plaque rupture detected by intravascular ultrasound. J Am Coll Cardiol. 2002; 40:904–910.
15. Hong YJ, Jeong MH, Choi YH, et al. Differences in intravascular ultrasound findings in culprit lesions in infarct-related arteries between ST segment elevation myocardial infarction and non-ST segment elevation myocardial infarction. J Cardiol. 2010; 56:15–22.
16. Kusama I, Hibi K, Kosuge M, et al. Impact of plaque rupture on infarct size in ST-segment elevation anterior acute myocardial infarction. J Am Coll Cardiol. 2007; 50:1230–1237.
17. Fujii K, Kobayashi Y, Mintz GS, et al. Intravascular ultrasound assessment of ulcerated ruptured plaques: a comparison of culprit and nonculprit lesions of patients with acute coronary syndromes and lesions in patients without acute coronary syndromes. Circulation. 2003; 108:2473–2478.
18. Nakamura M, Nishikawa H, Mukai S, et al. Impact of coronary artery remodeling on clinical presentation of coronary artery disease: an intravascular ultrasound study. J Am Coll Cardiol. 2001; 37:63–69.
19. Hasegawa T, Ehara S, Kobayashi Y, et al. Acute myocardial infarction: clinical characteristics and plaque morphology between expansive remodeling and constrictive remodeling by intravascular ultrasound. Am Heart J. 2006; 151:332–337.
20. Endo M, Hibi K, Shimizu T, et al. Impact of ultrasound attenuation and plaque rupture as detected by intravascular ultrasound on the incidence of no-reflow phenomenon after percutaneous coronary intervention in ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2010; 3:540–549.
21. Wu X, Mintz GS, Xu K, et al. The relationship between attenuated plaque identified by intravascular ultrasound and no-reflow after stenting in acute myocardial infarction: the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) trial. JACC Cardiovasc Interv. 2011; 4:495–502.
22. Lee SY, Mintz GS, Kim SY, et al. Attenuated plaque detected by intravascular ultrasound: clinical, angiographic, and morphologic features and post-percutaneous coronary intervention complications in patients with acute coronary syndromes. JACC Cardiovasc Interv. 2009; 2:65–72.
23. Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000; 20:1262–1275.
24. Libby P. Molecular bases of the acute coronary syndromes. Circulation. 1995; 91:2844–2850.
25. Rodriguez-Granillo GA, García-García HM, Mc Fadden EP, et al. In vivo intravascular ultrasound-derived thin-cap fibroatheroma detection using ultrasound radiofrequency data analysis. J Am Coll Cardiol. 2005; 46:2038–2042.
26. Hong MK, Mintz GS, Lee CW, et al. Comparison of virtual histology to intravascular ultrasound of culprit coronary lesions in acute coronary syndrome and target coronary lesions in stable angina pectoris. Am J Cardiol. 2007; 100:953–959.
27. Peterson ED, Jollis JG, Bebchuk JD, et al. Changes in mortality after myocardial revascularization in the elderly. The national Medicare experience. Ann Intern Med. 1994; 121:919–927.
28. De Gregorio J, Kobayashi Y, Albiero R, et al. Coronary artery stenting in the elderly: short-term outcome and long-term angiographic and clinical follow-up. J Am Coll Cardiol. 1998; 32:577–583.
29. Hassani SE, Mintz GS, Fong HS, et al. Negative remodeling and calcified plaque in octogenarians with acute myocardial infarction: an intravascular ultrasound analysis. J Am Coll Cardiol. 2006; 47:2413–2419.
30. Sano T, Tanaka A, Namba M, et al. C-reactive protein and lesion morphology in patients with acute myocardial infarction. Circulation. 2003; 108:282–285.
31. Tanaka A, Shimada K, Sano T, et al. Multiple plaque rupture and C-reactive protein in acute myocardial infarction. J Am Coll Cardiol. 2005; 45:1594–1599.
32. Bocksch W, Schartl M, Beckmann S, Dreysse S, Fleck E. Intravascular ultrasound imaging in patients with acute myocardial infarction. Eur Heart J. 1995; 16:Suppl J. 46–52.
33. Hong MK, Park SW, Lee CW, et al. Long-term outcomes of minor plaque prolapsed within stents documented with intravascular ultrasound. Catheter Cardiovasc Interv. 2000; 51:22–26.
34. Hong YJ, Jeong MH, Ahn Y, et al. Plaque prolapse after stent implantation in patients with acute myocardial infarction: an intravascular ultrasound analysis. JACC Cardiovasc Imaging. 2008; 1:489–497.
35. van der Hoeven BL, Liem SS, Dijkstra J, et al. Stent malapposition after sirolimus-eluting and bare-metal stent implantation in patients with ST-segment elevation myocardial infarction: acute and 9-month intravascular ultrasound results of the MISSION! intervention study. JACC Cardiovasc Interv. 2008; 1:192–201.
36. Kusama I, Hibi K, Kosuge M, et al. Impact of plaque rupture on infarct size in ST-segment elevation anterior acute myocardial infarction. J Am Coll Cardiol. 2007; 50:1230–1237.
37. Hong YJ, Jeong MH, Choi YH, et al. Positive remodeling is associated with more plaque vulnerability and higher frequency of plaque prolapse accompanied with post-procedural cardiac enzyme elevation compared with intermediate/negative remodeling in patients with acute myocardial infarction. J Cardiol. 2009; 53:278–287.
38. Hong YJ, Jeong MH, Choi YH, et al. Predictors of no-reflow after percutaneous coronary intervention for culprit lesion with plaque rupture in infarct-related artery in patients with acute myocardial infarction. J Cardiol. 2009; 54:36–44.
39. Ohshima K, Ikeda S, Kadota H, et al. Cavity volume of ruptured plaque is an independent predictor for angiographic no-reflow phenomenon during primary angioplasty in patients with ST-segment elevation myocardial infarction. J Cardiol. 2011; 57:36–43.
40. Kawaguchi R, Oshima S, Jingu M, et al. Usefulness of virtual histology intravascular ultrasound to predict distal embolization for ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2007; 50:1641–1646.
41. Kawamoto T, Okura H, Koyama Y, et al. The relationship between coronary plaque characteristics and small embolic particles during coronary stent implantation. J Am Coll Cardiol. 2007; 50:1635–1640.
42. Bae JH, Kwon TG, Hyun DW, Rihal CS, Lerman A. Predictors of slow flow during primary percutaneous coronary intervention: an intravascular ultrasound-virtual histology study. Heart. 2008; 94:1559–1564.
43. Nakamura T, Kubo N, Ako J, Momomura S. Angiographic no-reflow phenomenon and plaque characteristics by virtual histology intravascular ultrasound in patients with acute myocardial infarction. J Interv Cardiol. 2007; 20:335–339.
44. Daemen J, Wenaweser P, Tsuchida K, et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet. 2007; 369:667–678.
45. van Werkum JW, Heestermans AA, Zomer AC, et al. Predictors of coronary stent thrombosis: the Dutch stent thrombosis registry. J Am Coll Cardiol. 2009; 53:1399–1409.
46. Kimura T, Morimoto T, Nakagawa Y, et al. Antiplatelet therapy and stent thrombosis after sirolimus-eluting stent implantation. Circulation. 2009; 119:987–995.
47. Choi SY, Witzenbichler B, Maehara A, et al. Intravascular ultrasound findings of early stent thrombosis after primary percutaneous intervention in acute myocardial infarction: a Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) substudy. Circ Cardiovasc Interv. 2011; 4:239–247.
48. Hong YJ, Jeong MH, Choi YH, et al. Clinical, angiographic, and intravascular ultrasound predictors of early stent thrombosis in patients with acute myocardial infarction. Int J Cardiol. 2013; 168:1674–1675.
49. Nissen SE, Tuzcu EM, Schoenhagen P, et al. Statin therapy, LDL-cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med. 2005; 352:29–38.
50. Nissen SE, Nicholls SJ, Sipahi I, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA. 2006; 295:1556–1565.
51. Lehman SJ, Schlett CL, Bamberg F, et al. Assessment of coronary plaque progression in coronary computed tomography angiography using a semiquantitative score. JACC Cardiovasc Imaging. 2009; 2:1262–1270.
52. Nicholls SJ, Tuzcu EM, Wolski K, et al. Coronary artery calcification and changes in atheroma burden in response to established medical therapies. J Am Coll Cardiol. 2007; 49:263–270.
53. Hong YJ, Jeong MH, Choi YH, et al. Impact of baseline plaque components on plaque progression in nonintervened coronary segments in patients with angina pectoris on rosuvastatin 10 mg/day. Am J Cardiol. 2010; 106:1241–1247.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download