This article has been corrected. See "Erratum to: Additive Beneficial Effects of Valsartan Combined with Rosuvastatin in the Treatment of Hypercholesterolemic Hypertensive Patients" in Volume 45 on page 349.
Abstract
Background and Objectives
We compared the efficacy and safety of valsartan and rosuvastatin combination therapy with each treatment alone in hypercholesterolemic hypertensive patients.
Subjects and Methods
Patients who met inclusion criteria were randomized to receive 1 of the following 2-month drug regimens: valsartan 160 mg plus rosuvastatin 20 mg, valsartan 160 mg plus placebo, or rosuvastatin 20 mg plus placebo. The primary efficacy variables were change in sitting diastolic blood pressure (sitDBP) and sitting systolic blood pressure (sitSBP), and percentage change in low-density lipoprotein-cholesterol (LDL-C) in the combination, valsartan, and rosuvastatin groups. Adverse events (AEs) during the study were analyzed.
Results
A total of 354 patients were screened and 123 of them were finally randomized. Changes of sitDBP by least squares mean (LSM) were -11.1, -7.2, and -3.6 mm Hg, respectively, and was greater in the combination, as compared to both valsartan (p=0.02) and rosuvastatin (p<0.001). Changes of sitSBP by LSM were -13.2, -10.8, and -4.9 mm Hg, and was greater in the combination, as compared to rosuvastatin (p=0.006) and not valsartan (p=0.42). Percentage changes of LDL-C by LSM were -52, -4, and -47% in each group, and was greater in the combination, as compared to valsartan (p<0.001), similar to rosuvastatin (p=0.16). Most AEs were mild and resolved by the end of the study.
Hypertension and hyperlipidemia are well known cardiovascular risk factors that commonly coexist in a single patient.1) Patients with multiple risk factors have higher cardiovascular risk than patients with single factor.2) Thus it is critical to comprehensively control multiple factors for reducing future cardiovascular events. Angiotensin receptor blockers (ARBs) reduce cardiovascular events as well as blood pressure (BP), and are currently recommended as first line therapy for hypertension control.3) Previous studies have shown up-regulation of angiotensin II type 1 receptor by high levels of low-density lipoprotein-cholesterol (LDL-C),4) and low-density lipoprotein oxidation in patients with hypertension.5) Thus, it might be reasonable to use ARB and lipid-lowering agents, particularly a statin, for effective control of these 2 risk factors.
Valsartan, an ARB, has dose-dependent efficacy for lowering BP.6) Prior outcome studies have shown beneficial clinical effects of valsartan.7)8) Rosuvastatin is a strong efficacy statin, and the latest U.S. guideline recommends it for high intensive lipid-lowering therapy.9) It has low extra-hepatic tissue penetration, low potential for CYP3A4 interactions, and distinct advantage in substantial LDL-C lowering capacity.10) A few studies suggested that statins may improve vasodilation capacity of large artery, which can affect BP.11) Though clinical efficacy of ARB and statins for BP and LDL-C control have been studied before,12)13) no randomized clinical trial has examined combined valsartan and rosuvastatin treatment.
We investigated whether the combination of valsartan and rosuvastatin would show additional efficacy and acceptable safety, as compared to each component alone for patients with hypertension and hyperlipidemia.
Patients aged 20-80 years who had hypertension and hyperlipidemia were eligible for the study. At screening, patients who had been administered antihypertensive agents, or drug-naïve patients with sitting diastolic blood pressure (sitDBP) ≥90 mm Hg were included. Patients who had coronary artery disease (CAD) or CAD equivalents, and were classified as high risk according to National Cholesterol Education Program- Adult Treatment Panel (NCEP-ATP) III guidelines,14) were included if sitDBP was ≥80 mm Hg. At randomization, patients with sitDBP ≥90 mm Hg or high risk patients with sitDBP ≥80 mm Hg were finally enrolled and randomized into treatment groups.15) Lipid profile inclusion criteria included patients who had been administered lipid-modifying agents, or drug-naive patients who satisfied one of following: LDL-C≥100 mg/dL in patients with CAD, or CAD risk equivalents; LDL-C≥130 mg/dL in patients with ≥2 risk factors; or LDL-C≥160 mg/dL in patients with ≤1 cardiovascular risk factor. After therapeutic lifestyle change (TLC), patients who met the following criteria were randomized: ≥100 mg/dL in patients with CAD or CAD risk equivalents; LDLC≥ 130 mg/dL in patients with ≥2 risk factors with 10-year risk of 10-20%; LDL-C≥160 mg/dL in patients with ≤1 risk factor or ≥2 risk factors with 10-year risk <10%.
Exclusion criteria included sitting systolic blood pressure (sitSBP)≥180 mm Hg or sitDBP≥110 mm Hg (for the high risk group, sitSBP≥160 mm Hg or sitDBP≥100 mm Hg), LDL-C≥250 mg/dL, or TG≥400 mg/dL at screening and randomization (week 0); symptomatic orthostatic hypotension at screening (decrease in sitSBP≥20 mm Hg or decrease in sitDBP≥10 mm Hg); history of acute coronary syndrome within 6 months, percutaneous coronary intervention or coronary artery bypass graft, severe heart failure (functional class III or IV), severe valve disease, clinically significant arrhythmias; or stroke or transient ischemic attack within the last 6 months. Patients with severe obesity (body mass index≥40 kg/m2); uncontrolled diabetes mellitus (Hemoglobin A1c≥9.0%); thyroid dysfunction (TSH≥1.5xupper limit of normal [ULN]); creatine kinase (CK) >2×ULN; creatinine clearance <30 mL/min; serum creatinine ≥2 mg/dL; alanine transaminase (ALT) or aspartate aminotransferase (AST) ≥2×ULN; potassium <3.5 mEq/L or >5.5 mEq/L were also excluded.
This study was a multicenter, randomized, factorial, double-blind, double-dummy Phase III trial to evaluate the efficacy and safety of combination treatment with valsartan 160 mg and rosuvastatin 20 mg, as compared to each component alone in patients with hypertension and hyperlipidemia. The protocol was approved by the local Institutional Review Boards, and all subjects gave written informed consent at enrollment. The study was conducted in accordance with Korean Good Clinical Practice guidelines and the Declaration of Helsinki. This study was started on May 8th 2012 and finished on March 29th 2013.
Screening was conducted for patients who satisfied inclusion/exclusion criteria. Subjects who were taking antihypertensive or lipid-modifying agents discontinued those medications. Subjects who passed the screening tests underwent 6-week-TLC. After eligibility based on BP and lipid levels (week 0) was ascertained, subjects were randomly assigned to 1 of 3 treatment groups: combination therapy (valsartan 160 mg+rosuvastatin 20 mg), valsartan (valsartan 160 mg+placebo), or rosuvastatin (rosuvastatin 20 mg+placebo). Placebo was administrated during the treatment period to maintain double blindness. Randomization was performed by randomization tables prepared for each institution. After randomization, a code was transferred to a code manager who maintains the code in a separate file and breaks it in an emergency. However, no breaking of the code occurred in this study.
Subjects visited at the beginning of the 0th, 4th, and 8th weeks during the 8-week treatment period. Efficacy and safety were assessed at weeks 4 and 8. BP was measured by the same person at a regular time with a validated electronic sphygmomanometer (HEM-7080 IT, Omron Healthcare, Kyoto, Japan). Twelve hour (for patients without diabetes) or 8 hour (for patients with diabetes) fasting blood samples for lipid profile obtained during regular visits were analyzed in the central laboratory. Double blind protocol was maintained during the treatment period.
Major safety considerations leading to study treatment discontinuation were: uncontrolled high BP (e.g., sitSBP≥200 mm Hg, sitDBP≥120 mm Hg) or hypotension (e.g., sitSBP <90 mm Hg, sitDBP <60 mm Hg); ALT and AST >3×ULN; CK >5×ULN at any time during the study period.
Primary efficacy variables were change in sitDBP and sitSBP at week 8 for the combination and valsartan alone groups, and percentage change in LDL-C at week 8 for the combination and rosuvastatin alone groups. In addition, we analyzed changes of pulse pressure in each group. Secondary efficacy variables were proportion of subjects achieving BP treatment target at weeks 4 and 8 according to the European Society of Hypertension and European Society of Cardiology hypertension guideline (2003)15) and proportion of subjects achieving the LDL-C target at week 4 and 8 according to NCEP-ATP III guideline (2004).14)
Safety was based on the number of patients experiencing any adverse event (AE), or serious AE.
A serious AE refers to an AE that causes death or threatens life; causes short- or long-term hospitalization; causes permanent disability or reduction in function; causes congenital abnormality or a birth defect; or causes other medically important events. AEs and serious AEs were recorded at each study visit by history taking, physical examinations, and laboratory tests. The relationship of AEs to investigational products was evaluated.
The superiority of the combination therapy compared to each mono-therapy regarding changes of BP and LDL-C (change of sitDBP in the combination group vs. the rosuvastatin group and the % change of LDL-C in the combination group vs. the valsartan group) was tested in this study. For sample size calculation, 95% power was selected to each efficacy variable. Because two primary evaluation points needed to be proved at once, overall power became 90.25% (0.95×0.95=0.9025). Thirty-three patients per treatment group were needed to provide 95% power at a 2.5% confidence level (2-tailed). Differences in sitDBP and LDL-C changes were based on prior studies that used valsartan and rosuvastatin and assumed as -6.9 mm Hg16) and -49.7 mg/dL,17) respectively. Calculated sample size for sitDBP was greater than that for LDL-C and this was finally determined as sample size accordingly. To control for an expected dropout rate of 20%, 42 subjects were planned for each group. The full efficacy analysis set consisted of patients who had ≥1 primary efficacy endpoints and had received test agents at least once. The safety set, used for safety analysis, consisted of subjects who received test agents at least once after randomization.
Continuous variables were presented as mean and standard deviation. Frequency and percentage were presented for categorical variables. A test was conducted to determine any difference in baseline data between treatment groups. Analysis of variance or Kruskal-Wallis tests were conducted for continuous variables. Chisquare or Fisher's exact tests were conducted for categorical variables. The paired t-test was used to assess the difference in parameter before and after drug treatment within each group. Student's t-test was used to compare the mean value of continuous outcome variables between 2 groups. Analysis of covariance (ANCOVA) was conducted for the analysis of primary efficacy variables, with baseline levels as covariate. Outcome variables in the combination group were compared to those from the monotherapy group with the least squares mean estimated by ANCOVA for sitDBP change, sitSBP change, and LDL-C percentage change. A 2-tailed 95% confidence interval was calculated for the outcome variables. Prior to the ANCOVA, a test was performed for any interaction between the covariate and the treatment groups. If a significant interaction was present (p value<0.1), the corresponding covariate was excluded from the final ANCOVA model. All analyses were performed using SAS 9.2 (SAS Institute Inc., Cary, NC, USA).
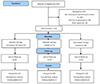
A total of 354 subjects from 23 institutions underwent screening, of whom 123 were randomized. 231 subjects were excluded after TLC. The number of exclusion before randomization was relatively large and mainly due to improvement of LDL-C or BP after 6-week TLC. One hundred and six and 74 subjects did not meet LDL-C level and BP, respectively. Thirty-four subjects declined to participate after submission of written consent. Of the randomized subjects, 101 completed the study: 35 in the combination group, 36 in the valsartan group, and 30 in the rosuvastatin group. Of the 123 randomized subjects, 121 were included in the safety set, while 116 were included in the analysis set (Fig. 1).
Characteristics of the subjects who were randomized were presented in Table 1. The mean age was 61.5 years and 68% were male. Most baseline variables were not different between groups. The mean sitDBP and mean LDL-C level of all subjects were 93±6 mm Hg and 153±28 mg/dL respectively, and were similar in the 3 groups (Table 1). Pulse pressure (p=0.04) showed significant (p=0.04), and sitSBP showed marginal difference (p=0.055) between the groups. The difference of sitSBP became significant in the full analysis set after a few subjects dropped out during the study.
Changes in outcome variables at week 8 were presented in Table 2. SitDBP changes by least square mean (LSM) were -11.1, -7.2, and -3.6 mm Hg in each group, respectively, and was significantly greater in the combination group, as compared to both valsartan (p=0.02) and rosuvastatin groups (p<0.001). Changes of sitSBP by LSM were -13.2, -10.8, and -4.9 mm Hg, respectively, and was greater in the combination group vs. rosuvastatin group (p=0.006), but not compared to valsartan group (p=0.42) (Fig. 2A). Percentage change of LDL-C by LSM were -52, -4, and -47% in each group, and was greater in the combination group, as compared to the valsartan group (p<0.001), but not compared to rosuvastatin group (p=0.16). In addition, there was no significant difference in terms of change in pulse pressure between the combination and valsartan group. (-1.3 mm Hg vs. -4.4 mm Hg, respectively, p=0.31) (Supplementary Table 1 in the online-only Data Supplement).
Percentage changes of LDL-C were -53±12% in the combination group and -46±16% in the rosuvastatin group. The difference between the 2 groups was not significant before and after ANCOVA adjustment (Fig. 2B). In the valsartan group, LDL-C levels did not significantly change through the 8-week treatment (-4±19%, p=0.065) (Table 2).
Proportion of subjects who achieved target BP at weeks 4 and 8 were shown in Fig. 3A. Proportions at week 4 were not different between the combination and valsartan groups (41.5% vs. 32.5%, respectively, p=0.40). Proportion at week 8 was higher, as compared to week 4 in the combination group without significance (51.2% vs. 35.0%, respectively, p=0.14).
Proportion of subjects who achieved target LDL-C level at weeks 4 and 8 were presented in Fig. 3B. Proportions at week 4 were similar in the combination and rosuvastatin groups. At week 8, proportion was marginally higher, but not significant, in the combination group, as compared to the rosuvastatin group (100% vs. 91.4%, respectively, p=0.09).
Ten (23%) in the combination group, 7 (17%) in the valsartan group, and 4 (11%) in the rosuvastatin group experienced AEs. Most AEs were mild and resolved at the end of the study (Table 3). Subjects who experienced AEs possibly related to test agents were: 1 in the combination group (2.3%), 2 in the valsartan group (4.9%), and 1 in the rosuvastatin group (2.7%). One subject in the combination group showed ALT and AST levels >3×ULN at week 8, which returned to normal at follow-up. In addition, one in the valsartan group had CK level >3×ULN at week 8, which became normal at follow-up.
One serious AE was a patient admitted on a day of randomization due to nausea and poor oral intake. However, this symptom was not likely to be associated with the test agents. In addition, they were both resolved after occurrence.
Combination of valsartan 160 mg and rosuvastatin 20 mg reduced sitDBP more than valsartan 160 mg alone by week 8 of treatment. sitSBP reduction was not significantly different between the 2 groups. Percentage change of LDL-C was similar between the combination and rosuvastatin groups. Target BP and LDL-C achievement rates were higher in the combination group than each group alone without statistical significance. Treatment regimens were well-tolerated during the study. These results demonstrated that this combination has an additive effect on BP lowering and is an appropriate therapeutic option for patients with hypertension and hyperlipidemia.
Several reports demonstrated effects of statin on BP in animal studies. Lovastatin and pravastatin attenuated the onset and progression of hypertension and reduced proteinuria and glomerular injury.18)19) A few studies showed that statins significantly lowered BP, as compared to placebo in untreated hypertensive patients.12)20) The Brisighella Heart Study evaluated the effect of different lipidlowering strategies on BP.21) Subjects with total cholesterol ≥239 mg/dL were treated with 1 of 4 lipid-lowering regimens: low-fat diet, cholestyramine, gemfibrozil, or simvastatin. After 5 years of treatment, BP decreased only in the group with SBP ≥140 mm Hg, and was greater with simvastatin treatment. Conversely, BP did not decrease in the groups with lower baseline BP. This study suggested that statins might improve BP control in subjects with both hypercholesterolemia and under-controlled hypertension.
Sposito et al.13) noted greater BP lowering with enalapril or lisinopril in a small group of patients treated with pravastatin or lovastatin. A similar effect for simvastatin was reported in patients under treatment with various antihypertensive medications.11)20) A retrospective analysis showed that effects of statin on BP were more obvious in patients treated with renin-angiotensin system blockades or calcium channel blockers.11) However, statins' additional antihypertensive effects are sometimes inconsistent. In one study, patients who received simvastatin 20 mg or 80 mg plus valsartan 160 mg for 12 weeks did not have lower BP, as compared to those who assigned to valsartan 160 mg alone.22) In the present study, additional BP lowering was noted only for diastolic BP and not for systolic BP. Although the precise reason for this finding cannot be fully understood, it is plausible that marginally higher baseline sitSBP in the valsartan group might have influenced the results. A prior study demonstrated that statin shows BP lowering effect, particularly in under-controlled hypertension. Accordingly, in our study, the combination therapy could not have shown its additional BP lowering effect in sitSBP, because the baseline sitSBP was relatively lower in the combination group, as compared to the valsartan group. To adjust the baseline difference, we used ANCOVA for primary efficacy variables with baseline levels as covariate. Prior to ANCOVA, a test for interaction between the covariate and the treatment groups showed no interaction. In the manuscript, we presented the least-square mean change of the primary efficacy variables by ANCOVA.
In addition, the difference in target BP achievement rate between the 2 groups was not statistically significant. However, we cannot rule out a statistically greater BP lowering in the combination group with a study of a larger sample size.
The mechanism by which statins affect blood pressure is not clearly understood. One possibility is that statins' antihypertensive effect is dependent on their ability to prevent renal vascular hypertrophy. Another explanation is that statins improve endothelium dependent vaso-relaxation,23) which was more prominent in a higer dose.24) Peripheral arterial compliance can be impaired in patients with high blood cholesterol levels, and this might affect BP.25) Statins improve vasodilatory capacity, and possibly increase artery compliance. In addition, it has been experimentally shown that statins may promote up-regulation of vascular nitric oxide synthase.26) This could contribute to modulation of peripheral vascular tone, irrespective of reduction in circulating cholesterol levels. An additional mechanism may be the capacity of statins to blunt vasoconstrictive and presser response to vascular agonists, such as angiotensin II or norepinephrine.27) Interestingly, all of these conditions might increase the sensitivity of the vessel wall to the vasodilating effect of medications such as angiotensin-converting enzyme inhibitors or ARBs. Recently, several studies have shown that the combination of renin-angiotensin system blockades and statins had distinct vascular effects in hypertension with dyslipidemia. These studies demonstrated that this combination improves vascular function or metabolic phenotypes like adiponectin levels or insulin sensitivity.28)29)
To the best our knowledge, this randomized, double-blind study is the first to show the additive effect of valsartan and rosuvastatin combination therapy on BP. Because these 2 agents are now popularly prescribed based on clinical evidence, our data further supports that this combination is a reasonable regimen. However, this study had a relatively small sample size and was conducted over a short period of time. Thus, further clinical studies over a longer period are needed to confirm this combination's clinical benefit. It was beyond the scope of the current study to explore potential mechanisms of the 2 agents' additive effect. Further studies on the biological mechanisms, such as vascular function, might be helpful in understanding our results. The randomization process was strictly conducted in our study with the support of a statistics specialist. However, marginal imbalance in the baseline characteristics seemed to occur by chance. Imbalance in the baseline characteristics is known to occur even in qualified randomized trials. Moreover, such imbalance reportedly does not affect the internal validity of studies.30)
This study found greater BP reduction in combination of valsartan 160 mg and rosuvastatin 20 mg than valsartan 160 mg, and comparable LDL-C lowering to rosuvastatin 20 mg. This result indicated that a combination of these 2 agents might be a reasonable therapeutic option for patients with hypertension and hyperlipidemia.
Figures and Tables
Fig. 2
BP change in the combination and valsartan groups at week 8 (A). Percentage LDL-C change in the combination and rosuvastatin groups at week 8 (B). Error bars represent standard error. BP: blood pressure, sitDBP: sitting diastolic blood pressure, sitSBP: sitting systolic blood pressure, LDLC: low-density lipoprotein-cholesterol.

Fig. 3
Percentage of subjects achieving treatment goal. Percentage achieving ESH-ESC guideline (2003) BP target (A). Percentage achieving LDL-C NCEP-ATP III guideline (2004) target (B). ESH-ESC: European Society of Hypertension and European Society of Cardiology, BP: blood pressure, LDC-C: low-density lipoprotein-cholesterol, NCEP-ATP: National Cholesterol Education Program-Adult Treatment Panel.

Table 1
Baseline characteristics of the study subjects

Table 2
Changes of blood pressure and LDL-C at week 8

Values are mean±SD. *Comparison between combination vs. valsartan groups, †Comparison between combination vs. rosuvastatin groups, ‡Comparison in a group before and after treatment. LDL-C: low-density lipoprotein-cholesterol, sitDBP: sitting diastolic blood pressure, LSM: least squares mean, sitSBP: sitting systolic blood pressure
Acknowledgments
This study was supported by LG Life Science. The sponsor provided investigational products including the active and placebo pills used in this study. J.H.J and Y.L.J are full-time employees of LG Life. Yangsoo Jang and Sang-Hak Lee contributed to the study design, interpretation of the data, and writing of the manuscript. Jae-Hyeon Juhn and YeiLe Jung contributed to the statistical analyses. Ji-Yong Jang contributed to the interpretation of the data and writing of the manuscript. All authors contributed to the design of the article and revised the manuscript. We thank all study participants, investigators, and coordinators.
References
1. Kannel WB. Blood pressure as a cardiovascular risk factor: prevention and treatment. JAMA. 1996; 275:1571–1576.
2. Thomas F, Bean K, Guize L, Quentzel S, Argyriadis P, Benetos A. Combined effects of systolic blood pressure and serum cholesterol on cardiovascular mortality in young (<55 years) men and women. Eur Heart J. 2002; 23:528–535.
3. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014; 311:507–520.
4. Nickenig G, Harrison DG. The AT(1)-type angiotensin receptor in oxidative stress and atherogenesis: Part II: AT(1) receptor regulation. Circulation. 2002; 105:530–536.
5. Keidar S, Kaplan M, Shapira C, Brook JG, Aviram M. Low density lipoprotein isolated from patients with essential hypertension exhibits increased propensity for oxidation and enhanced uptake by macrophages: a possible role for angiotensin II. Atherosclerosis. 1994; 107:71–84.
6. Pool JL, Glazer R, Chiang YT, Gatlin M. Dose-response efficacy of valsartan, a new angiotensin II receptor blocker. J Hum Hypertens. 1999; 13:275–281.
7. Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003; 349:1893–1906.
8. Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004; 363:2022–2031.
9. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014; 63:2889–2934.
10. White CM. A review of the pharmacologic and pharmacokinetic aspects of rosuvastatin. J Clin Pharmacol. 2002; 42:963–970.
11. Borghi C, Prandin MG, Costa FV, Bacchelli S, Degli Esposti D, Ambrosioni E. Use of statins and blood pressure control in treated hypertensive patients with hypercholesterolemia. J Cardiovasc Pharmacol. 2000; 35:549–555.
12. Glorioso N, Troffa C, Filigheddu F, et al. Effect of the HMG-CoA reductase inhibitors on blood pressure in patients with essential hypertension and primary hypercholesterolemia. Hypertension. 1999; 34:1281–1286.
13. Sposito AC, Mansur AP, Coelho OR, et al. Additional reduction in blood pressure after cholesterol-lowering treatment by statins (lovastatin or pravastatin) in hypercholesterolemic patients using angiotensin-converting enzyme inhibitors (enalapril or lisinopril). Am J Cardiol. 1999; 83:1497–1499.
14. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. 2001; 285:2486–2497.
15. Cifkova R, Erdine S, Fagard R, et al. Practice guidelines for primary care physicians: 2003 ESH/ESC hypertension guidelines. J Hypertens. 2003; 21:1779–1786.
16. Philipp T, Smith TR, Glazer R, et al. Two multicenter, 8-week, randomized, double-blind, placebo-controlled, parallel-group studies evaluating the efficacy and tolerability of amlodipine and valsartan in combination and as monotherapy in adult patients with mild to moderate essential hypertension. Clin Ther. 2007; 29:563–580.
17. Avis HJ, Hutten BA, Gagné C, et al. Efficacy and safety of rosuvastatin therapy for children with familial hypercholesterolemia. J Am Coll Cardiol. 2010; 55:1121–1126.
18. Wilson TW, Alonso-Galicia M, Roman RJ. Effects of lipid-lowering agents in the Dahl salt-sensitive rat. Hypertension. 1998; 31:225–231.
19. Jiang J, Roman RJ. Lovastatin prevents development of hypertension in spontaneously hypertensive rats. Hypertension. 1997; 30:968–974.
20. Nazzaro P, Manzari M, Merlo M, et al. Distinct and combined vascular effects of ACE blockade and HMG-CoA reductase inhibition in hypertensive subjects. Hypertension. 1999; 33:719–725.
21. Borghi C, Dormi A, D'Addato S, et al. Trends in blood pressure control and antihypertensive treatment in clinical practice: the Brisighella Heart Study. J Hypertens. 2004; 22:1707–1716.
22. Rajagopalan S, Zannad F, Radauceanu A, et al. Effects of valsartan alone versus valsartan/simvastatin combination on ambulatory blood pressure, C-reactive protein, lipoproteins, and monocyte chemoattractant protein-1 in patients with hyperlipidemia and hypertension. Am J Cardiol. 2007; 100:222–226.
23. O'Driscoll G, Green D, Taylor RR. Simvastatin, an HMG-coenzyme A reductase inhibitor, improves endothelial function within 1 month. Circulation. 1997; 95:1126–1131.
24. Egede R, Jensen LO, Hansen HS, et al. Effect of intensive lipid-lowering treatment compared to moderate lipid-lowering treatment with rosuvastatin on endothelial function in high risk patients. Int J Cardiol. 2012; 158:376–379.
25. Lewis TV, Cooper BA, Dart AM, Chin-Dusting JP. Responses to endothelium-dependent agonists in subcutaneous arteries excised from hypercholesterolaemic men. Br J Pharmacol. 1998; 124:222–228.
26. Kaesemeyer WH, Caldwell RB, Huang J, Caldwell RW. Pravastatin sodium activates endothelial nitric oxide synthase independent of its cholesterol-lowering actions. J Am Coll Cardiol. 1999; 33:234–241.
27. Straznicky NE, Howes LG, Lam W, Louis WJ. Effects of pravastatin on cardiovascular reactivity to norepinephrine and angiotensin II in patients with hypercholesterolemia and systemic hypertension. Am J Cardiol. 1995; 75:582–586.
28. Savić V, Eržen B, Janić M, et al. Improvement of arterial wall characteristics by the low-dose fluvastatin and valsartan combination in type 1 diabetes mellitus patients. Diab Vasc Dis Res. 2013; 10:420–425.
29. Koh KK, Quon MJ, Han SH, et al. Additive beneficial effects of losartan combined with simvastatin in the treatment of hypercholesterolemic, hypertensive patients. Circulation. 2004; 110:3687–3692.
30. Roberts C, Torgerson DJ. Understanding controlled trials: baseline imbalance in randomized controlled trials. BMJ. 1999; 319:185.
Supplementary Materials
The online-only Data Supplement is available with this article at http://dx.doi.org/10.4070/kcj.2015.45.3.225.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download