This article has been corrected. See "Erratum to: Small Left Atrial Size Complicating Percutaneous Transcatheter Device Closure of Secundum Atrial Septal Defect with Conventional Approach" in Volume 45 on page 348.
Abstract
Background and Objectives
Transcatheter device closure becomes the first option for treating secundum atrial septal defect (ASD), but the conventional method is sometimes unsuccessful even when the defect size indicates the closure to be feasible. To increase the success rate, modified methods have been introduced and used. This study aimed to find predictors for using the modified methods in the device closure of secundum ASDs.
Subjects and Methods
Between October 2010 and December 2012, 92 patients with ASDs underwent the transcatheter device closure. We analyzed the sizes of the defect, the surrounding rims, and the ratios of the left atrium (LA) dimensions to the device size in the patients who underwent the procedure either using the conventional or modified methods.
Results
Among the 88 successful cases (95.7%), 22 patients (25%) required modified methods (12 using pulmonary vein and 10 using balloon). The modified method group had the larger size of ASDs and smaller posterosuperior rim. The mean ratios of the LA anteroposterior diameter, width, and length to the device size were all significantly smaller in the modified methods group than in the conventional group (1.20 vs. 1.56, 1.32 vs. 1.71, and 1.61 vs. 2.07, respectively). We found that the risk factors for the modified methods were smaller retroaortic rim, larger ASD, and smaller LA dimension/device size.
Advances in percutaneous transcatheter device closure of secundum atrial septal defect (ASD) have resulted in high success rates.1)2) Nowadays, it is becoming a primary option for treating secundum ASD because transcatheter closures may avoid the complications associated with open-heart surgery and can reduce the complication rates and the length of the hospital stay, compared with surgical repairs.3)4) However, certain patients still require surgical repairs, even when the defect size indicates that transcatheter closures are feasible. It has been reported that the risk factors for the failure of transcatheter closure included the patient being a small child, having a large-sized single defect, and having a deficient surrounding the rims.5) For such difficult cases, modified transcatheter techniques have been recently introduced. For example, balloon assisted (BA) method and methods using pulmonary vein (PV) have improved the success rates and overcame the anatomical disadvantages, but those methods still have limitations with varying success rates.3)4)6)
To find the risk factors for the complicated conventional method of the transcatheter device closure, we investigated the echocardiographic parameters for the anatomy of ASD and for the size of the left atrium (LA).
This study analyzed 92 consecutive patients who had undergone percutaneous transcatheter closure of the secundum ASD at the congenital heart diseases center at the Asan Medical Center, Seoul, Korea between October 2010 and December 2012. The median age of the study group was 3.5 years (range, 0.9-57.4 years).We excluded cases of combined cardiac anomalies, except for mild valvar pulmonary stenosis, multiple ASDs except cribriform ASD, or associated syndromes. The medical records of the patients were retrospectively reviewed. Age at intervention, sex, weight, height, and 2D images of the pre-interventional transthoracic echocardiography (TTE) were analyzed with a post-processing program by Image Arena (Tomtech Imaging Systems, Munich, Germany). The defect sizes were measured by intraoperative transesophageal echocardiography (TEE) and the sizes of device used were also collected from the catheterization reports. The institutional review board at Asan Medical Center approved this study. Informed consents were waived due to the retrospective study design.
Prior to each intervention, each patient had been admitted and examined by a single physician through a TTE using an iE33 with a S5-1 transducer (Philips Medical Systems, Andover, MA, USA) after resting at least 15 minutes in the supine position. Children under 3 years old were sedated with chloral hydrate (0.5 cc/kg) while monitoring the vital signs and oxygen saturation, at room air.
The rims around the secundum ASD were measured in 3 planes and were defined as follows: superior vena cava and inferior vena cava rim from a subcostal view, posterosuperior (PS) and mitral valve rim from an apical four-chamber (A4C) view, and posteroinferior and retroaortic (RAo) rim from a parasternal short axis view (Fig. 1). The LA dimensions were measured at the end of the systolic phase; the anteroposterior diameter (AP) was measured in a parasternal longaxis view. The width was measured in an A4C view. The mean value of the length was calculated from the lengths measured in A4C and apical two chamber (A2C) views. The LA volume was calculated by the length and areas of the LA in the A4C and A2C views {biplane area-length method: 0.85×(AreaA2C)×(AreaA4C)/DL}.
The transcatheter device closure was performed under general anesthesia, and the secundum ASD size was repeatedly measured by an intraoperative TEE by a single physician who was different from the TTE-examiner with an iE33 machine (Philips Medical Systems, Andover, MA, USA). Prior to the cardiac catheterization, anticoagulation was initiated with heparin (100 units/kg, intravenously) and a standard right cardiac catheterization was performed for a hemodynamic study. We did not measure the balloon that routinely stretched the defect size except when a large aneurysmal interatrial septum was present. All the patients underwent transcatheter closure with the Amplatzer septal occluder (AGA Medical Corporation, Golden Valley, MN, USA) and device implantation was initially tried by the conventional method that had already been extensively described.3)4) Devices measuring1-2 mm larger than the defect sizes were measured by an intraoperative TEE and were employed in the transcatheter closure. If the device implantation was not successful after multiple attempts using the conventional method, the so-called 'PV method' was attempted as Papa et al.4) reported. The PV method is described as follows: after the sheath was located in the right upper PV, the LA disc was deployed with the remaining unspread and stretched from the orifice of PV through the LA cavity until the right atrial disc was delivered and contacted to the vicinity of interatrial septum from the right atrial side. By pushing the cable with the device, the LA disc fell out of the PV and spread automatically and fully with an approximation to the interatrial septum4) (Fig. 2A and B).
If neither the conventional nor the PV method was successful, we tried the BA method next, as Kammache et al.6) reported through another transvenous approach (usually in the opposite side femoral vein) for peripheral balloon insertion. The supporting wire for the balloon was located into either the right or left PV depending upon the locations of the deficient rims. The sheath for the device was located on the other side. We preferred to use peripheral balloons instead of the bigger test-occlusion ones. When a balloon was introduced through the wire and positioned at the interatrial septum, the LA disc that was 'assisted' or supported by the inflating balloon was delivered first. This procedure prevented the prolapse of the LA disc by keeping the plane of the LA disc parallel to the interatrial septum. The right atrial disc was deployed in this position, leaving the device in a dumbbell shape. The subsequent deflation of the balloon approximated the disc towards the interatrial septum. By carefully steadying the device position, the balloon and wire were extracted. After the successful removal of the balloon and the wire, we confirmed the device position and the lack of the atrial shunt using the TEE, prior to the final release of the device (Fig. 2C and D).
If the device implantation was unsuccessful after all three methods, we aborted further attempts at the percutaneous transcatheter closure. Devices were not deployed and extracted in cases of unstable positioning with either a Minnesota wiggling, a compression of the mitral valve or aortic wall, or significant residual leakage during the TEE evaluation. Patients, whose percutaneous transcatheter closure was unsuccessful, underwent surgical closure at a later time.
An antiplatelet agent (aspirin 3.5-5.0 mg/kg once daily) was prescribed for six months following all successful closures. Device stability, residual shunt, or compression of the cardiac valve and aortic wall were evaluated at post-interventional day 1, 6 months, and 12 months, using the TTE. We also evaluated any clinical symptoms and signs of the patients in the outpatient clinic.
We used the Statistical Package for Social Science (SPSS) for Windows, version 18 (SPSS Inc., Chicago, IL, USA) for the statistical analysis. Student ttest and Mann-Whitney test for continuous variables and chi-square test for categorical variables were used for comparing the groups, as appropriate. The logistic regression test was used and receiver-operating curves were plotted for the cut-off values. The p value <0.05 was considered significant.
A percutaneous transcatheter closure had been attempted in a total of 92 patients during this study's period. Successful closures at each stage are summarized as a flow chart (Fig. 3). Sixty-six patients (71.7%) underwent successful closures by the conventional method, and 22 patients (23.9%) underwent successful closures by the PV (n=12, 13.0%) and BA method (n=10, 10.9%). A percutaneous closure was unsuccessful in four patients (4.3%), who subsequently underwent surgical repair. We divided the successful patients into the conventional method group and the modified methods group, including the PV and BA method. The demographic data of the conventional and modified methods groups are characterized in Table 1. There were no significant differences in age, height, weight, and body surface area between the groups.
The mean values of the two groups' anatomical parameters of ASDs by echocardiography are shown in Table 2. We found that the modified methods group had the smaller PS rim (6.1 mm vs. 7.7 mm) and larger ASD (18.1 mm vs. 13.8 mm). The mean sizes of the RAo rim of both groups were deficient (<5 mm) and were not significantly different. The LA diameters and LA volumes indexed by body surface area were also similar in the two groups. Table 3 shows the anatomical risk factors for using the modified risk factors from the result of the logistic regression test. Larger ASD and smaller RAo rim were found to be significant factors for the modified methods group.
We examined the LA dimensions/device size because we postulated that the relatively large size of the LA disc might technically hinder the device implantation with conventional method. The ratios of the LA dimensions (AP, width, and length) to the device sizes were significantly smaller in the modified group compared to the conventional method group (p=0.001). Fig. 4 illustrates the statistical differences, in the ratios of the LA dimensions to the employed device size, between the conventional and modified methods groups. The mean values of the LA AP diameter/device size were 1.56 in the conventional method group and 1.20 in the modified methods group, which was significantly smaller (p=0.001). The mean LA width/device size and LA length/device size were also smaller in the modified methods group (1.32 vs. 1.71 and 1.61 vs. 2.07, respectively). Among the three ratios, the LA AP diameter/device size were the most important parameter based on the result of the logistic regression test (odds ratio 0.059, 95% confidence interval 0.008-0.448, p=0.006). Comparing the two modified methods (PV and BA) groups, the BA group showed significantly larger sizes of ASD and smaller LA diameters/Amplatzer septal occluder sizes (p=0.031 and p=0.027, respectively). The receiver-operating characteristic curve showed the cut-off values for the three ratios (Fig. 5). For the cut-off values for using the conventional methods with the LA AP diameter/device size, the LA width/device size and LA length/device size might be 1.69, 1.64 and 1.78, respectively (95.0% of specificity and 46.6% of sensitivity).
When we reviewed the four patients who failed to have the percutaneous closure, Fig. 6 illustrates the only LA AP diameter/device that was significantly smaller than those of the successful cases (p=0.031) while the LA width/device size and LA length/device size were not significantly smaller than those of the successful cases (p=0.066 and 0.443, respectively). The 3 of 4 failed cases showed that the LA AP diameters/device sizes were below 1 and that the LA lengths were smaller than the LA disc of the device.
There was no peri-procedural complication, such as device displacement, sustained arrhythmia, or vascular complications, within the initial 24-hours follow-up during the study period. No early complication, such as aortic erosion or thrombosis, (within the first 6 months) after the hospital discharge was observed in any of the patients during the outpatient follow-up period.
This study showed an overall success rate of 95.7% for the transcatheter device closure of the secundum ASD and these results were comparable to other reports.2)3)5) Among the successful closures, the conventional method accounted for 85%, with the remaining 15% being closed with the modified methods, such as the PV or BA method. Because the latter group represented a considerable portion of patients, regarded as technically challenging cases, we tried to identify the predictors for these technical challenges prior to an intervention, possibly helping to prepare and to shorten the procedure time.7)
This study found two main predictors. One was the anatomical characteristic of a larger defect and smaller retroaortic rim, agreeing with previous reports.4)8),9),10) Large ASDs and the locations and the extent of deficient surrounding the rims were previously known to be risk factors of the modified methods.8),9),10) Papa et al.4) reported that the method using PV was helpful in patients with a deficient PS rim. This study showed that a larger defect and smaller RAo rim were the most powerful risk factors, although the modified methods group had a smaller PS rim. The RAo rim size, which was known to be unrelated to the success rate,8) was one of the risk factors for using the modified methods. These anatomical parameters should be precisely measured; however, we sometimes had difficulty in applying them to the pediatric population because the pre-interventional TEE is not a routine procedure in every center. The clinical cut-off values of the defect and surrounding rim size were not reported in children whose body surface area widely varied according to age and hemodynamic status.
The second predictor was the ratio of the LA dimensions to the employed device sizes. At times, we experienced a deformation of the left atrial disc into an abnormal shape in a small LA and it was frequently prolapsed into the right atrium. This resulted not from the LA size but from the relationship with the LA size and device size; this conclusion was supported by the fact that the indexed LA dimensions and LA volumes were not significantly different between the two groups. Because we postulated that the instability of the LA disc in a relatively small LA might be a risk factor for using the modified methods, resulting in the right atrium disc to be settled down first, we found that the LA dimensions/device size were significantly smaller in patients who failed with the conventional method. Those ratios that were not influenced by the patient's body surface area could be easily applicable to the pediatric population. Because we measured the LA dimensions from the three standard TTE planes, the ratios can be calculated prior to the intervention, possibly helping to predict the success rate or to screen for the surgical cases. The cut-off values of the LA diameters (AP, width, and length)/device size for using conventional methods were 1.64, 1.69, and 1.78, respectively. High specificity (95.0%) and low sensitivity (46.6%) were important limitations in our study; however, because this study was designed retrospectively, these cut-off values with very high specificity could be one of the screening parameters for using the modified methods or for the risk of failure. Because these values showed good specificity (95%) and low sensitivity (46.6%), we should prepare modified methods pre-interventionally when the ratios are below the cutoff levels. For example, if the LA diameter/device size ratio is under 1.6, we might prepare for the technical challenges in case of the failure of the conventional method and we could explain the risk.
When we analyzed the four failed cases, 3 of 4 patients (75%) showed the LA AP diameter/device size was very small (<1.0) and we could consider surgical correction after much more experience with combining a meticulous intraoperative TEE data for the surrounding rims. We could not find a possible explanation for one case, who had a 24 mm-defect size and 1 deficient RAo rim. For the screening of the surgical cases, the interatrial septal length might be considered because 75% of the failed cases had an interatrial septal length that was shorter than the LA disc length. However, we needed more data and we did not reach a statistical significance in the success rate when we compared the LA disc length and LA length parallel to the interatrial septal length.
Our strategy of the transcatheter device closure was that the conventional method was attempted first in all of the cases, and, if unsuccessful, we used the PV and BA methods. The BA method was reserved as the last procedure, as it required an additional vascular access. According to this study, we sometimes secured another vascular access prior to anticoagulation and this procedure might reduce the chance of vascular and bleeding complications. Additionally, we modified the BA method described by Dalvi and his colleagues3) by using smaller peripheral balloons with smaller sheath because we did not use the usual occlusion balloon measuring size of ASD.11),12),13) This could reduce vascular complications related to the use of larger sheaths.14)
Our study had some limitations. First of all, the number of patients in this study was quite small and additional data should be collected for future studies. The case control study could be better explained for analyzing the impact of the small LA dimension as a single risk factor for using the modified methods and also needs a larger study population. Because of the study design and procedure strategy, the cut-off values for using the modified methods could only discriminate the apparent case because of low sensitivity. We do not recommend using the modified methods for the initial attempt based only on the LA dimension. However, our procedure strategy could make up for it. While evaluating cases from a single operator potentially reduced an inter-operator bias, this limited the generalization of the study results to only one center. Although the peri-procedural and acute complication were absent during the short term of the study period, we did not evaluate the long-term complications reported in other studies.15),16),17),18)
Figures and Tables
Fig. 1
Measurements of the LA diameters (A, B, C) and schematic diagram of the surrounding rims from the pre-interventional TTE (D). A: AP, anteroposterior diameter measured at the midline of the LA from a PLAX view during an end-systolic phase (red). B: parameters measured from an A4C view at an end systolic phase; W, width (blue); LA4C, length (yellow); AA4C, area measured from an A4C view (sky blue). C: parameters measured from an A2C at an end systolic phase; LA2C, length (yellow); AA2C, area measured from an A2C (sky blue). D: gray regions represent the surrounding rims measured from a subcoastal, an A4C, and a PSAX view. LA: left atrium, TTE: transthoracic echocardiography, PLAX: parasternal long axis, A4C: apical four chambers, A2C: apical two chambers, PSAX: parasternal short axis, FR: frame rate, C: compression rate, P: persistent grade, HGen: general harmony, BPM: beat per minute, ASD: secundum atrial septal defect, TV: tricuspid valve.
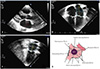
Fig. 2
Pulmonary vein method and schematic diagram. Angiographic film showed the approximation of the RA disc by pushing the cable while the LA disc was in the orifice of the right upper PV (A). Schematic diagram of the PV method (B). Modified balloon-assisted method and schematic diagram. Angiographic film showed both wings of the device were deployed before implantation by supporting the peripheral balloon (C). Schematic diagram of BA method (D). RA: right atrium, LA disc of the device was prevented from prolapsed into the RA by balloon (arrow head). LA: left atrium, PV: pulmonary vein, BA: balloon assisted.

Fig. 3
Flow chart of the strategy with treating ASD patients. All the patients started with the conventional method and then the modified methods were used; the BA method was the last approach after the PV method failed. If all three methods failed, surgical closure was indicated. ASD: secundum atrial septal defect, BA: balloon assisted; PV: pulmonary vein.
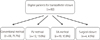
Fig. 4
Comparisons of the two groups in the ratios of the LA diameters (AP, width, and length) to the ASO size. A: ratio of LA AP diameter to ASO size. B: ratio of LA width to ASO size. C: ratio of LA length to ASO size. Pink box represented conventional group and blue box represented modified group. *Significant with p=0.001. LA: left atrium, AP: anteroposterior, ASO: Amplatzer septal occluder.

Fig. 5
ROC curve for the ratios of the LA AP diameter (red), LA width (blue), and LA length (yellow) to the ASO size. Areas under the curve were 0.719 (p=0.004), 0.716 (p=0.04), and 0.716 (p=0.004), respectively. ROC: receiveroperating characteristic, LA: left atrium, AP: anteroposterior, ASO: Amplatzer septal occluder, W: width, L: length

Fig. 6
Comparisons between the failed and successful cases in the modified methods group. A: ratio of LA AP diameter to ASO size. B: ratio of LA width to ASO size. C: ratio of LA length to ASO size. Black circle represented each of the failed cases and the blue circle represented each of the successful cases in the ratios of the LA diameters (AP, W and L) to the ASO size. LA: left atrium, AP: anteroposterior, ASO: Amplatzer septal occluder, W: width, L: length

Table 1
Demographic data of the study groups

Table 2
Comparison of the echocardiography parameters between the conventional method and modified methods group

Values are mean±SD. *Significant. SVC: superior vena cava, IVC: inferior vena cava, PS: posterosuperior, MV: mitral valve, PI: posteroinferior, RAo: retroaortic rim, ASD: secundum atrial septal defect, TEE: transesophageal echocardiography, LA: left atrium, DAP (at PLAX): anteroposterior diameter measured at a parasternal long axis, DW (at A4C): left atrium width measured at an apical four chamber, DL (at A4C): left atrium length measured at an apical four chamber
Table 3
Parameters associated with using the modified methods

References
1. Rosas M, Zabal C, Garcia-Montes J, Buendia A, Webb G, Attie F. Transcatheter versus surgical closure of secundum atrial septal defect in adults: impact of age at intervention. A concurrent matched comparative study. Congenit Heart Dis. 2007; 2:148–155.
2. Du ZD, Hijazi ZM, Kleinman CS, Silverman NH, Larntz K. Amplatzer Investigators. Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: results of a multicenter nonrandomized trial. J Am Coll Cardiol. 2002; 39:1836–1844.
3. Dalvi BV, Pinto RJ, Gupta A. New technique for device closure of large atrial septal defects. Catheter Cardiovasc Interv. 2005; 64:102–107.
4. Papa M, Gaspardone A, Fragasso G, et al. Feasibility and safety of transcatheter closure of atrial septal defects with deficient posterior rim. Catheter Cardiovasc Interv. 2013; 81:1180–1187.
5. Du ZD, Koenig P, Cao QL, Waight D, Heitschmidt M, Hijazi ZM. Comparison of transcatheter closure of secundum atrial septal defect using the Amplatzer septal occluder associated with deficient versus sufficient rims. Am J Cardiol. 2002; 90:865–869.
6. Kammache I, Mancini J, Ovaert C, Habib G, Fraisse A. Feasibility of transcatheter closure in unselected patients with secundum atrial septal defect, using Amplatzer devices and a modified sizing balloon technique. Catheter Cardiovasc Interv. 2011; 78:665–674.
7. Wahab HA, Almossawy A, Al Bitar I, Hijazi ZM. Tips and tricks to prevent prolapse of the Amplatzer septal occluder through large atrial septal defects. Catheter Cardiovasc Interv. 2011; 78:1041–1044.
8. Huang CF, Fang CY, Ko SF, et al. Transcatheter closure of atrial septal defects with superior-anterior rim deficiency using Amplatzer septal occluder. J Formos Med Assoc. 2007; 106:986–991.
9. Butera G, Romagnoli E, Carminati M, et al. Treatment of isolated secundum atrial septal defects: impact of age and defect morphology in 1,013 consecutive patients. Am Heart J. 2008; 156:706–712.
10. Park SJ, Kim NK, Kim JO, Yoo BW, Choi JY, Sul JH. Morphologic characteristics and relating factors to the need of technical modification in transcatheter closure of large atrial septal defect (>/=25 mm). Korean Circ J. 2010; 40:191–196.
11. Hijazi ZM. Device closure of secundum atrial septal defects: to balloon size or not to balloon size. Ann Pediatr Cardiol. 2011; 4:34–35.
12. Carlson KM, Justino H, O'Brien RE. Transcatheter atrial septal defect closure: modified balloon sizing technique to avoid overstretching the defect and oversizing the Amplatzer septal occluder. Catheter Cardiovasc Interv. 2005; 66:390–396.
13. Rigatelli G, Dell'avvocata F, Cardaioli P, et al. Safety and long-term outcome of modified intracardiac echocardiography-assisted "noballoon" sizing technique for transcatheter closure of ostium secundum atrial septal defect. J Interv Cardiol. 2012; 25:628–634.
14. Wiley JM, White CJ, Uretsky BF. Noncoronary complications of coronary intervention. Catheter Cardiovasc Interv. 2002; 57:257–265.
15. Chessa M, Carminati M, Butera G, et al. Early and late complications associated with transcatheter occlusion of secundum atrial septal defect. J Am Coll Cardiol. 2002; 39:1061–1065.
16. Abaci A, Unlu S, Alsancak Y, Kaya U, Sezenoz B. Short and long term complications of device closure of atrial septal defect and patent foramen ovale: meta-analysis of 28,142 patients from 203 studies. Catheter Cardiovasc Interv. 2013; 82:1123–1138.
17. Crawford GB, Brindis RG, Krucoff MW, Mansalis BP, Carroll JD. Percutaneous atrial septal occluder devices and cardiac erosion: a review of the literature. Catheter Cardiovasc Interv. 2012; 80:157–167.
18. Raghuram AR, Krishnan R, Kumar S, Balamurugan K. Complications in atrial septal defect device closure. Interact Cardiovasc Thorac Surg. 2008; 7:167–169.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download