See the reply "Takotsubo Cardiomyopathy related to Pheochromocytoma or Other Etiology Should Be Considered as Similar" in Volume 45 on page 535.
Abstract
Background and Objectives
Excessive catecholamine causes the alteration of cardiac structure and function. This study evaluated if there is any difference in left ventricular hypertrophy (LVH) and QTc prolongation in conditions with pheochromocytoma and Takotsubo cardiomyopathy (TC).
Subjects and Methods
We reviewed the medical records of 20 pheochromocytoma patients for cardiovascular events prior to diagnosis. The patient's clinical history and electrocardiographic and echocardiographic findings were compared to those of 20 patients diagnosed with TC.
Results
Left ventricular (LV) mass index (133.3±37.8 vs. 113.3±17.3, p=0.031), relative wall thickness (0.55±0.15 vs. 0.47±0.07, p=032) and elevated blood pressure (BP) were more prominent in pheochromocytoma compared to TC. The mean creatinine kinase-MB elevation, reduced LV systolic function and ST segment changes were more prominent in the TC group compared to the pheochromocytoma groups (all p<0.05). The prevalence of QTc prolongation was high in patients with pheochromocytoma (45%) and TC (55%), and TC male patients appeared to have a more prolonged QTc interval. Urine epinephrine (r=0.844, p=0.004) and norepinephrine level (r=0.782, p=0.013) were significantly correlated with LV mass index, and the predictors for the QTc prolongation were male gender and the presence of LVH.
Pheochromocytoma is a rare catecholamine-producing tumor with a highly variable clinical presentation.1) Pheochromocytoma presentation may include numerous cardiovascular manifestations and is usually identified with prevalent cardiac manifestations or paroxysmal hypertension associated with other signs and symptoms of catecholamine excess.2)3) Although sinus tachycardia with palpitations is the most prevalent cardiac rhythm abnormality, pheochromocytoma can induce more serious arrhythmias or conduction disturbances, such as prolongation of the QTc interval and ventricular tachycardia followed by cardiac arrest.4)
Although QTc interval prolongation in pheochromocytoma is rare, it has been reported in several cases, and it has been proposed that catecholamine excess may contribute to its development.5)6)7) Recently, more attention has been given to the increasing number of cases of Takotsubo cardiomyopathy (TC).8)9) The relationship between TC and abnormal repolarization has been well-documented, which is probably because systolic dysfunction is associated with both TC and QT-interval prolongation.10) QT interval prolongation and ST-T changes occur during the acute or subacute phase of illness, and these electrocardiography (ECG) changes typically normalize within several weeks of left ventricular (LV) wall motion improvement.10) However, the systolic function and wall motion abnormality are usually normal in pheochromocytoma; therefore, the difference in QTc interval prolongation between pheochromocytoma and TC is not evaluated.
We hypothesized that there would be a difference in left ventricular hypertrophy (LVH) or QTc interval induced by pheochromocytoma, chronic catecholamine excess, TC, and acute transient catecholamine excess. To explore this hypothesis, we reviewed the initial cardiac manifestations of pheochromocytoma prior to diagnosis and compared the results to patients with TC without pheochromocytoma.
We enrolled 20 consecutive patients with pheochromocytoma, who exhibited cardiovascular events prior to the diagnosis at Kosin University Gospel Hospital between January 2002 and July 2013, and 20 consecutive patients diagnosed as TC without pheochromocytoma during the same period for the comparison. Patients with the following conditions were excluded from the study: another cause of secondary hypertension, diabetes mellitus, angina pectoris, prior myocardial infarction, chronic obstructive lung disorder, severe renal failure, significant ST-T changes, and coronary artery disease. We reviewed the medical records for the clinical history, biochemical test values, ECG, and echocardiographic findings to identify initial manifestations.
Pheochromocytoma was diagnosed based on a high concentration of metanephrine or normetanephrine in a 24-hour urine sample. Enhanced computed tomography was used to identify adrenal and extra-adrenal tumors, and an I-131 metaiodobenzylguanidine tumor single-photon emission computed tomography was used to visualize mass uptake. The histopathology of the mass was used to create a final confirmation after surgical resection of the tumor. TC was diagnosed if the patient presented with an acute coronary syndrome after intense psychological stress. This diagnosis was also used in clinical manifestations and for regional wall motion and ECG abnormalities that were disproportionate to the degree of cardiac biomarker elevation with normal coronary angiography.
The LV mass was calculated using the corrected American Society of Echocardiography cube formula,11) and the value was indexed for body surface area to obtain the LV mass index (LVMI). The relative wall thickness (RWT) was measured as the ratio between double posterior wall thicknesses to the LV diastolic cavity diameter at end diastole. LVH was defined as increased LVMI (>104 g/m2 in women and >116 g/m2 in men).12) Concentric hypertrophy was defined as LVH with increased RWT (>0.43). Concentric LV remodeling was defined as increased RWT with a normal LVMI. Eccentric hypertrophy was defined as increased LVMI with a normal RWT. Bazett's formula was used to calculate the QTc values, and an abnormal QTc in males was defined as a QTc above 450 ms and above 470 ms in females.13)
Statistical analyses were performed with the statistical program Statistical Package for the Social Sciences for Windows version 12.0 (SPSS Inc., Chicago, IL, USA). Results are presented as the mean±standard deviation or percentage. The difference between the categorical variables was determined using the χ2-test. The independent Student's t-test was used to determine the difference in normally distributed data, and the Mann-Whitney U test was used for comparison of median for the non-parametrically distributed variables. A linear association was examined using the Pearson's correlation test for parametric data and the Spearman correlation test for the non-parametric data. Multivariate linear regression analysis was used to determine the predictors for QTc prolongation in catecholamine excess stress, using age, heart rate, gender, LVH, and LV ejection faction (EF) as covariates. Statistical significance was set as p<0.05.
The initial cardiac manifestations of pheochromocytoma were labile hypertension (50%), chest pain (20%), syncope (10%), dizziness (10%), and palpitations (10%) (Table 1). The tumor size, serum, and 24-hour urine catecholamine levels of pheochromocytoma are shown in Table 2. All patients had elevated 24-hour urine metanephrine and normetanephrine levels, variable levels of serum epinephrine and urine norepinephrine, and tumor size was correlated with 24-hour urine metanephrine (r=0.792, p<0.001). The precedent stressors for TC patients were surgery, emotional stress, and infection; the stressors were usually accompanied with symptoms, such as chest pain and dyspnea. The majority (90%) of the TC patients showed ST-segment changes (Table 3). Two male patients (10%) with pheochromocytoma showed apical ballooning with LVH, and 15 patients (75%) from the pheochromocytoma group and 19 TC patients (95%) underwent coronary angiography, which revealed that none of the patients had significant stenotic lesions in their coronary arteries. The analysis indicated that the pheochromocytoma group was younger than the TC group, and there were more female TC patients (Table 4). The mean creatinine kinase (CK)-MB elevation, reduced LV EF and ST-segment changes were more prominent in the TC group compared to the pheochromocytoma groups (all p<0.05) (Table 4). The prevalence of QTc prolongation was high in both pheochromocytoma (45%) and TC (55%), and the prevalence was higher in men (pheochromocytoma: male 50%, female 40%; TC: male 100%, female; 31%). There were no correlations between QTc interval and the serum and urine catecholamine levels. However, the TC male patients appeared to have a more prolonged QTc interval (TC: male 499.3±33.3 msec, female 474.5±45.5 msec; pheochromocytoma: male 463.0±45.7, female 461.±71.8 msec).
Left ventricular mass index (133.3±37.8 vs. 113.3±17.3; p=0.031), RWT (0.55±0.15 vs. 0.47±0.07; p=032), and blood pressure (BP) elevation were more prominent in pheochromocytoma compared to those of TC group. All male with pheochromocytoma exhibited a concentric LVH pattern, whereas female pheochromocytoma patients showed various LVH patterns (normal 40%, concentric remodeling 40%, eccentric hypertrophy 10%, and concentric hypertrophy 10%) (Table 1). The LV geometry was relatively normal in TC patients (males: normal 57%, eccentric hypertrophy 29%, concentric hypertrophy 14%; females: normal 54%, concentric hypertrophy 31%, eccentric hypertrophy 15%) (Table 3).
Although serum epinephrine and norepinephrine levels were not correlated with LVMI, urine epinephrine (r=0.844, p=0.004) and norepinephrine level (r=0.782, p=0.013) were significantly correlated with LVMI. The results indicated that serum epinephrine level was only significantly correlated with RWT (r=0.862, p=0.003) (Table 5).
We estimated the predictors for the QTc prolongation in multivariable linear regression analyses using age, heart rate, gender, LVH, and LV EF as covariates. Male gender {odds ratio (OR); 4.50, 95% confidence interval (CI), 1.17-17.37, p=0.029} and the presence of LVH (OR; 4.89, 95% CI, 1.20-19.94, p=0.027) showed significant associations with the presence of QT prolongation in catecholamine excess state.
This comparative retrospective study demonstrates the prominence of QTc prolongation in patients with catecholamine excess, pheochromocytoma, and TC, regardless of BP and systolic LV function. LVH was more prominent in pheochromocytoma with chronic catecholamine excess; however, acute catecholamine surge might have a role in the electrical remodeling. The independent associations for the QTc prolongation were male gender and the presence of LVH.
Pheochromocytoma is a rare tumor that produces catecholamines and has manifestations that can vary from no significant symptoms to sudden cardiac death (SCD).14) Previous studies showed various initial pheochromocytoma manifestations prior to greater numbers of hypertension diagnoses,15)16) and reported cardiac complications of pheochromocytoma include hypertrophic cardiomyopathy, dilated cardiomyopathy, arrhythmia, myocardial ischemia, and SCD.17)18) Acute complications may contribute to additional cardiac problems, such as LV dysfunction and TC, and several reports have demonstrated that coronary angiography usually reveals no stenosis or obstruction, mimicking TC, in pheochromocytoma patients.8)9)19)
Chronic myocardial hypoxia and metabolic myocarditis of hypercatecholaminemia are known causes of pheochromocytoma cardiomyopathy.20) Chronic elevated levels of circulating plasma norepinephrine in pheochromocytoma patients can produce structural and functional LV remodeling, including LVH. Although some authors have shown a positive association between plasma norepinephrine level and LVH,21)22) other observational studies have demonstrated that the majority of patients with pheochromocytoma have a normal LV mass.23)24) However, our results showed that there was a predominantly increased LVMI with RWT in male patients with pheochromocytoma, and some patients with TC also exhibited LVH. Systolic and diastolic BP were also higher in male patients with pheochromocytoma with an abnormal LVH pattern, which implies that chronic catecholamine excess produced by an underlying tumor may affect men more than women. Our results, although limited by a small sample size, might suggest that norepinephrine serves as a synergistic partner with arterial hypertension in the pathogenesis of cardiac hypertrophy, even with a transient surge, which might be attenuated by estrogen. Although our results showed no significant association between plasma norepinephrine level and LVMI, the significant positive correlation of urine epinephrine and norepinephrine levels with LVMI suggests that the local delivery of catecholamines to receptors may be more important than their passage through the circulation to induce LVH.
Electrocardiographic abnormalities that include rhythm, conduction and repolarization may occur in patients with pheochormocytoma.25)26) Several cases have reported that QT interval prolongation could be related to pheochromocytoma,5)6)7) and we also experienced a 48-year-old pheochromocytoma woman with marked QT interval prolongation (Fig. 1A) that progressed to torsades de pointes (Fig. 1B), which returned to normal (Fig. 1C) after right adrenal glandectomy. Initially, she was diagnosed with long QT syndrome with torsades de pointes followed by cardiac arrest. However, her 24-hour urinary biochemical analysis showed elevated metanephrine and normetanephrine, and the patient was confirmed as pheochromocytoma. Paroxysmal repolarization mechanisms, such as marked prolongation of the QT interval, remain unclear; however, higher levels of catecholamines may affect cardiac injury and induce longer QTc interval changes.27) QT interval prolongation is also common in the subacute phase of TC and other stress-induced cardiomyopathies,28) and presently there were some differences that could be used to differentiate between these situations. QTc interval prolongation in addition to deep and wide symmetrical T wave inversion, LV systolic dysfunction, and CK-MB elevation were more prominent in the TC group compared to the pheochromocytoma group, which indicated that acute catecholamine release impact on the heart was more dominant in the TC group. This result may also suggest that acute catecholamine surges could cause intracellular calcium overloading and disturb repolarization, which could prolong the QTc interval. Interestingly, our results showed no significant association between catecholamine level and QTc interval, and in some cases of acute catecholamine surges in pheochromocytoma, a much longer QT interval with ST changes occurred independent of catecholamine level or tumor size. Although limited by the retrospective nature of the study, the other interesting finding was that the presence of LVH and male gender were significantly related with the QTc prolongation, indicating that the impact of catecholamine excess on ventricular repolarization might be more pronounced in men. A previous study reported that during metal stress, men react with a more pronounced effect on ventricular electrophysiological properties.29) This difference might be one contributing factor explaining why women are less prone to certain ventricular arrhythmias and SCD than are men. Moreover, similar to our results, a recent study showed that prolonged QTc was independently associated with SCD among subjects with LVH, and merits further evaluation as a predictor of SCD in LVH.30)
The major limitations of this study were the retrospective design and the small sample size. Because pheochromocytoma is a rare disease and the recruited number of patients in this single-center study may have been insufficient, so the differences in manifestations between different sexes or between serum and urine may not represent the general result. There are also significant differences of age, gender proportion, BP as well EF, which could also influence LV geometry and QTc prolongation in both groups. It would be more reasonable to compare the clinical and echocardiographic characteristics of TC to the pheochromocytoma with reduced EF (TC-like cardiomyopathy) or compare the pheochromocytoma to TC with LVH. However, our intention was to compare the difference between chronic catecholamine excess and acute transient catecholamine excess. So, we believe the limitation does not attenuate the clinical meaning of the study. Because our study was a cross-sectional, retrospective study, the causative nature of the LV geometric pattern associations, QTc interval, and catecholamine level or duration could not be established. Moreover, the serum and urine catecholamine assessment was not performed with our TC group. Further studies with a larger patient population and prospective studies that compare pheochromocytoma and TC should be performed to address the limitations of this study.
However, in spite of these limitations, this study demonstrates that cardiac manifestations other than hypertension could be critical initial manifestations of pheochromocytoma, which is important for future diagnoses. Increased LVH with high BP and a prolonged QTc interval, especially in relatively young males with cardiac symptoms and without obvious lesions, could be indicative of underlying pheochromocytoma. Finally, based on the results of this study, unnecessary interventions can be avoided, and cardiac manifestations with an underlying disease can be diagnosed and successfully treated.
Figures and Tables
Fig. 1
Electrocardiography (ECG) of a patient with prominent QTc prolongation. The patient complained of intermittent palpitation and diaphoresis with syncope for 4 years. A: her ECG on admission showed normal sinus rhythm with a prolonged QTc interval (596 msec). B: two days after admission, a sudden onset of chest pain and syncope with loss of consciousness occurred abruptly, and ECG monitoring indicated torsades de pointes. C: after right adrenal gland removal, her electrocardiography returned to normal.
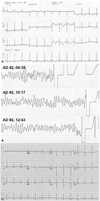
Table 1
Clinical variables of pheochromocytoma in 20 cases focusing on electrocardiogram and left ventricular hypertrophy

Table 2
Tumor size, characterization and serum and 24-hour-urine catecholamine levels of 20 pheochromocytoma cases

Table 3
Clinical variables of stress induced cardiomyopathy in 20 cases focusing on cardiac manifestations

Table 4
Comparison of clinical, echocardiographic and electrocardiographic parameters between pheochromocytoma and Takotsubo cardiomyopathy (TC)

All values are mean±SD. BP: blood pressure, LVEDD: left ventricular end diastolic dimension, LVESD: left ventricular end systolic dimension, IVSd: interventricular dimension at diastole, PWTd: posterior thickness at diastole, RWT: relative wall thickness, LVMI: left ventricular mass index, LVD: left ventricular hypertrophy, EF: ejection fraction, CK-MB: creatine kinase-MB
Table 5
Correlations among the serum and 24-hour-urine catecholamine levels, blood pressure and left ventricular hypertrophy and QTc interval of 20 pheochromocytoma cases

References
1. Whalen RK, Althausen AF, Daniels GH. Extra-adrenal pheochromocytoma. J Urol. 1992; 147:1–10.
2. Amar L, Servais A, Gimenez-Roqueplo AP, Zinzindohoue F, Chatellier G, Plouin PF. Year of diagnosis, features at presentation, and risk of recurrence in patients with pheochromocytoma or secreting paraganglioma. J Clin Endocrinol Metab. 2005; 90:2110–2116.
3. Prejbisz A, Lenders JW, Eisenhofer G, Januszewicz A. Cardiovascular manifestations of phaeochromocytoma. J Hypertens. 2011; 29:2049–2060.
4. Leite LR, Macedo PG, Santos SN, Quaglia L, Mesas CE, De Paola A. Life-threatening cardiac manifestations of pheochromocytoma. Case Rep Med. 2010; 2010:976120.
5. Sacha J, Wester A, Hordynski G, Pluta W. QT interval prolongation during ECG evolution in takotsubo cardiomyopathy poses a threat of torsade de pointes to predisposed patients. Case report of a female patient with congenital AV block. Herz. 2013; 38:790–795.
6. van der Heide K, de Haes A, Wietasch GJ, Wiesfeld AC, Hendriks HG. Torsades de pointes during laparoscopic adrenalectomy of a pheochromocytoma: a case report. J Med Case Rep. 2011; 5:368.
7. Chakraborty P, Bhattacharjeee HK, Anandaraja S. Palpitition, presyncope and abdominal mass. Indian Heart J. 2010; 62:447–448.
8. Takizawa M, Kobayakawa N, Uozumi H, et al. A case of transient left ventricular ballooning with pheochromocytoma, supporting pathogenetic role of catecholamines in stress-induced cardiomyopathy or takotsubo cardiomyopathy. Int J Cardiol. 2007; 114:e15–e17.
9. Agarwal V, Kant G, Hans N, Messerli FH. Takotsubo-like cardiomyopathy in pheochromocytoma. Int J Cardiol. 2011; 153:241–248.
10. Matsuoka K, Okubo S, Fujii E, et al. Evaluation of the arrhythmogenecity of stress-induced "Takotsubo cardiomyopathy" from the time course of the 12-lead surface electrocardiogram. Am J Cardiol. 2003; 92:230–233.
11. Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986; 57:450–458.
12. Ganau A, Devereux RB, Roman MJ, et al. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992; 19:1550–1558.
13. Hannan PJ, Crow RS. Concerning the units for the QT interval corrected by Bazett's formula. Circulation. 1997; 96:3799.
14. Gifford RW Jr, Bravo EL, Manger WM. Diagnosis and management of pheochromocytoma. Cardiology. 1985; 72:Suppl 1. 126–130.
15. Giavarini A, Chedid A, Bobrie G, Plouin PF, Hagège A, Amar L. Acute catecholamine cardiomyopathy in patients with phaeochromocytoma or functional paraganglioma. Heart. 2013; 99:1438–1444.
16. Zelinka T, Petrák O, Turková H, et al. High incidence of cardiovascular complications in pheochromocytoma. Horm Metab Res. 2012; 44:379–384.
17. Eisenhofer G, Rivers G, Rosas AL, Quezado Z, Manger WM, Pacak K. Adverse drug reactions in patients with phaeochromocytoma: incidence, prevention and management. Drug Saf. 2007; 30:1031–1062.
18. Bybee KA, Prasad A. Stress-related cardiomyopathy syndromes. Circulation. 2008; 118:397–409.
19. Kaese S, Schülke C, Fischer D, Lebiedz P. Pheochromocytoma-induced takotsubo-like cardiomyopathy and global heart failure with need for extracorporal life support. Intensive Care Med. 2013; 39:1473–1474.
20. Radtke WE, Kazmier FJ, Rutherford BD, Sheps SG. Cardiovascular complications of pheochromocytoma crisis. Am J Cardiol. 1975; 35:701–705.
21. Kelm M, Schäfer S, Mingers S, et al. Left ventricular mass is linked to cardiac noradrenaline in normotensive and hypertensive patients. J Hypertens. 1996; 14:1357–1364.
22. Simpson P. Norepinephrine-stimulated hypertrophy of cultured rat myocardial cells is an alpha 1 adrenergic response. J Clin Invest. 1983; 72:732–738.
23. Fouad-Tarazi FM, Imamura M, Bravo EL, et al. Differences in left ventricular structural and functional changes between pheochromocytoma and essential hypertension. Role of elevated circulating catecholamines. Am J Hypertens. 1992; 5:134–140.
24. Shub C, Cueto-Garcia L, Sheps SG, Ilstrup DM, Tajik AJ. Echocardiographic findings in pheochromocytoma. Am J Cardiol. 1986; 57:971–975.
25. Goldbaum TS, Henochowicz S, Mustafa M, Blunda M, Lindsay J Jr. Pheochromocytoma presenting with Prinzmetal's angina. Am J Med. 1986; 81:921–922.
26. Cheng TO, Bashour TT. Striking electrocardiographic changes associated with pheochromocytoma. Masquerading as ischemic heart disease. Chest. 1976; 70:397–399.
27. Yu R, Nissen NN, Bannykh SI. Cardiac complications as initial manifestation of pheochromocytoma: frequency, outcome, and predictors. Endocr Pract. 2012; 18:483–492.
28. Ahn JH, Park SH, Shin WY, et al. Long QT syndrome and torsade de pointes associated with Takotsubo cardiomyopathy. J Korean Med Sci. 2011; 26:959–961.
29. Insulander P, Vallin H. Gender differences in electrophysiologic effects of mental stress and autonomic tone inhibition: a study in health individuals. J Cardiovasc Electrophysiol. 2005; 16:59–63.
30. Panikkath R, Reinier K, Uy-Evanado A, et al. Electrocardiographic predictors of sudden cardiac death in patients with left ventricular hypertrophy. Ann Noninvasive Electrocardiol. 2013; 18:225–229.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download