Abstract
Paravalvular leaks (PVLs) often occur after surgical valve replacement. Surgical reoperation has been the gold standard of therapy for PVLs, but it carries a higher operative risk and an increased incidence of re-leaks compared to the initial surgery. In high surgical risk patients with appropriate geometries, transcatheter closure of PVLs could be an alternative to redo-surgery. Here, we report a case of successful staged transcatheter closures of a fistula tract between the aorta and right atrium, and mitral PVLs after mitral valve replacement and tricuspid annuloplasty.
Paravalvular leaks (PVLs) are not a rare complication after surgical valve replacement. Approximately 1-5% of patients undergoing mitral or aortic valve replacement experience some sort of PVLs with varying clinical importance, ranging from asymptomatic to life-threatening.1) Redo-surgery has been the gold standard of therapy for PVLs. However, it is associated with a higher surgical risk and an increased risk for recurrent PVLs compared to the initial surgery.2)3)
Transcatheter closure of PVLs was first described in 1992 by Hourihan et al.4) Since then, this technique has evolved with various technical improvements, becoming an alternative to redo-surgery in high risk patients with appropriate defect geometries.1)2)5) Here we describe successful staged transcatheter closures for the fistula tract between the aorta and right atrium, and mitral PVL after mitral valve replacement and tricuspid annuloplasty.
A 71 year-old man was admitted due to exertional dyspnea (New York Heart Association functional class III). He had a past medical history of pericardiectomy due to effusive-constrictive pericarditis 29 years ago and mitral valve replacement with tricuspid annuloplasty due to severe mitral and tricuspid regurgitation 11 years ago. His electrocardiogram showed atrial fibrillation with controlled ventricular rhythm. Transthoracic echocardiography showed abnormal shunt flow from the aorta to the right atrium, a tissue defect in the mitral annulus with moderate PVL (distal jet area of 7 cm2), mild tricuspid regurgitation with mild resting pulmonary hypertension (maximal velocity of tricuspid regurgitation jet=3.2 m/s), and an enlarged left ventricle with near normal systolic function (end-diastolic dimension of the left ventricle=67 mm, ejection fraction=51%). Transesophageal echocardiography (TEE) also revealed continuous abnormal shunt flow through a fistula tract from the aorta (noncoronary cusp) to the right atrium (Fig. 1), and about a 4-5 mm sized tissue defect in the mitral annulus with moderate PVL (Fig. 2). On computed tomography, about a 3 mm PVL was observed at the lateral aspect of the mitral annulus (Fig. 3). His calculated logistic EuroSCORE and STS risk score were 16.7% and 7.0%, respectively. After discussion with both the surgical and interventional teams, we decided to perform staged transcatheter closures for both the fistula tract and mitral PVL.
The first procedure was conducted under general anesthesia with fluoroscopic and TEE guidance. A 7 French (Fr) sheath was inserted through the right femoral vein and 6 Fr sheaths were placed in both femoral arteries. A 6 Fr pigtail catheter was inserted through the right femoral artery. After a peripheral angiogram with a pigtail catheter, we confirmed the existence of a fistula tract between the aorta and right atrium. The fistula tract could be crossed with a 260 cm long 0.032 inch Terumo wire, and it was confirmed by TEE. A 6 Fr Cournand catheter was advanced into and passed through the defect. Another 6 Fr Cournand catheter was introduced into the right atrium (Fig. 4A). Using a Curry intravascular retriever, the guidewire was captured and withdrawn through the right femoral sheath. The guidewire was exchanged for a 0.035 inch stiff wire using a 6 Fr Cournand catheter. The 6 Fr Cournand catheter was replaced with an 8 Fr delivery sheath that was advanced into the ascending aorta. Because the sizes of the tissue defect on TEE were 9 mm in length and 4 mm in width, we selected an 8/6 mm sized Amplatzer duct occluder. After proper device positioning, which was confirmed using fluoroscopy and TEE, the device was deployed (Figs. 4B and 5A). Immediate TEE showed a well-positioned device, and disappearance of the abnormal shunt flow between the aorta and right atrium (Fig. 5B).
After the first procedure, his symptoms improved slightly and a follow-up echocardiography two months after the first procedure showed slightly decreased left ventricular diastolic dimension with reduced ejection fraction (from 51% to 46%) and persistent resting pulmonary hypertension. Percutaneous closure of the PVL was attempted to decrease left ventricular volume overloading. Under general anesthesia, a 7 Fr sheath was inserted through the right femoral vein and a 6 Fr sheath was inserted through the right femoral artery. A TEE was also used for guidance. A pigtail catheter was placed into the posterior cusp of the aortic valve through the right femoral artery. The sheath in the right femoral vein was exchanged for a Mullin sheath and dilator, which was advanced over a 0.032-inch guidewire into the superior vena cava. Thereafter, the guidewire was removed and a Brockenbrough needle was gently advanced to within a few millimeters of the tip of the dilator, and the needle was flushed and connected to a manifold for continuous pressure monitoring. With fluoroscopic guidance (RAO 45 degrees), the interatrial septum was punctured at the site of the fossa ovalis. The Mullin sheath was advanced into the left atrium. The stainless steel wire was crossed over the tissue defect in the mitral annulus. Using a 6 Fr multipurpose catheter, the stainless steel wire was exchanged for a 0.035 inch stiff wire (Fig. 6). After several dilatations with a septal dilator, the 8 Fr delivery sheath was placed in the left ventricle. Because the defect size on the TEE was about 4-5 mm, we also chose an 8/6 mm sized Amplatzer duct occluder. After proper device positioning was confirmed on the TEE, the device was deployed (Fig. 6). The TEE that was performed immediately afterwards showed a well-positioned device and a trivial PVL (Fig. 7). At 24 hours post-procedure, transthoracic echocardiography also showed well-positioned devices and no remnant shunt flow or PVL. Echocardiography immediately after the procedure showed persistent left ventricular dysfunction (ejection fraction=47%) but disappearance of resting pulmonary hypertension.
Repair of PVLs is only indicated when symptoms occur. Clinically significant PVLs most often occur in association with mitral prostheses, and less often with aortic, and only rarely with pulmonary or tricuspid prostheses.3) PVL may be related to heavy annular calcification, localized infection, tissue friability, suture rupture or suturing technique.2)6)7) Although reoperation has been the treatment of choice, this is associated with high morbidity and mortality. So, alternatives to surgical closure for PVLs in order to avoid the risk of reoperation have been pursued. Percutaneous repair is a feasible option in high-surgical-risk patients with appropriate geometries.3)7) However, the feasibility of percutaneous closure has to be assessed by defining the shape, size and location of the defect by performing echocardiography with three-dimensional defect reconstruction.8)
The patient presented above had a relatively high risk for surgery because the calculated logistic EuroSCORE and STS risk score were 16.7% and 7.0%, respectively, and he had already undergone cardiac surgery twice. In addition, on TEE and computed tomography, the location seemed to be favorable for percutaneous closure and the shape of the PVL was cylindrical. Therefore, we thought complete occlusion of the PVL would be possible by using an Amplatzer duct occluder available in Korea. And we performed percutaneous closure of the fistula tract from the aorta to the right atrium as the first procedure because this lesion was hemodynamically more significant. Although the cause of the fistula between the aorta and right atrium was uncertain, an injury related to the tricuspid annuloplasty was regarded as a possible etiology.
Percutaneous closure of PVLs is becoming more popular in recent days. Successful transcatheter closure of PVLs requires correct diagnosis, optimal device selection and monitoring during the procedure.7)9) PVLs must be assessed regarding the shape, size and site. Combined two-dimensional and three-dimensional echocardiographic examinations allow for correct non-invasive assessment and characterization of PVL. As conventional two-dimensional echocardiographic imaging alone can be misleading, three-dimensional echocardiography also plays a major role in procedural guidance.9)10) And selection of the most appropriate device and size for repair of PVL is critical for its success. The device for PVL is chosen based on the identified geometry of the defect as well as the surrounding structures. In the past, due to lack of dedicated devices, various types of coils and occluders have been used to treat PVLs.7)9)11) As most PVLs are not cylindrical, the oval shape of the Amplatzer Vascular Plug III may better fit crescent shapes. Today, this device is considered the first choice in the majority of cases.7)12) And transcatheter closure of PVLs requires a catheterization laboratory setup with enhanced imaging capabilities including fluoroscopy and TEE, especially three-dimensional TEE. The procedure is typically performed under general anesthesia for patient comfort and airway control due to the need for periprocedural TEE.9) Percutaneous repair of mitral paravalvular defects can be performed with antegrade, retrograde or transapical access. Selection of the access site depends on the location of the prosthesis, the location of the defect in relation to the valve, the presence of mechanical valves, and operator experience.1)7-9) In our case, we selected the antegrade approach via femoral venous access with interatrial transseptal puncture. The mitral paravalvular defect was successfully closed by an Amplatzer duct occluder. Previously performed percutaneous closure of the fistula tract from the aorta to the right atrium was also successful and free from complications.
The technical success rate has been reported to be variable, between 50% and 100%.7) Though rarely reported, there are many potential complications including the usual complications associated with general anesthesia, TEE, vascular access, and transseptal puncture. The most common complication associated with repair of PVL is bleeding. Other procedural specific complications are valve dehiscence, device embolization, infection of the local access site, infective endocarditis, impingement of valve leaflet motion and hemolysis. The risk for emergent surgery and for death as a complication of percutaneous repair is 1-2%.7)9) In spite of these complications, percutaneous closure for PVLs may still be safer than repeated surgery.
The safety and efficacy of percutaneous repair of PVL has never been compared to reoperation in a randomized trial. However, with technical improvement, transcatheter closure of PVL is likely to play an increasingly important role in the future. In conclusion, transcatheter closure of PVLs can be an effective, lower risk alternative to reoperation.
Figures and Tables
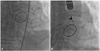
Fig. 1
Transesophageal echocardiographic images showing shunt flow from the aorta to the right atrium. Shunt flow was evident on color Doppler flow mapping (A), and the diameter of the fistula (arrows) was 4 mm (B). Ao: aorta, LA: left atrium, RA: right atrium, RV: right ventricle.
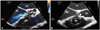
Fig. 2
Transesophageal echocardiographic images showing paravalvular leakage. Pathologic paravalvular mitral regurgitation jet (arrow) was evident at the lateral mitral annulus on color Doppler flow mapping (A), and the defect size was 4 mm (B). LA: left atrium, LV: left ventricle.

Fig. 3
Computed tomographic images showing paravalular leakage. The maximal defect size was 3 mm (A) and reconstructed images for the cardiac surgeons from the left atrial side showed that the defect was located at the antero-lateral mitral annulus (arrow, B).
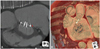
Fig. 4
Fluoroscopic images showing the procedure. A 6 Fr Cournand catheter was advanced into and passed through the defect from the aorta to the right atrium, and another 6 Fr Cournand catheter was introduced into the right atrium (A). An 8/6 mm sized Amplatzer duct occluder was deployed in the fistula tract from the aorta to the right atrium (B).
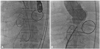
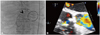
Fig. 5
Immediately after the procedure, both fluoroscopy (A) and transesophageal echocardiography (B) showed a well-positioned device and no remnant shunt (arrows).
References
1. Kursaklioglu H, Barcin C, Iyisoy A, Baysan O, Celik T, Kose S. Percutaneous closure of mitral paravalvular leak via retrograde approach: with use of the Amplatzer duct occluder II and without a wire loop. Tex Heart Inst J. 2010; 37:461–464.
2. Kuehl M, Schreieck J, Burgstahler C. Percutaneous closure of a periprosthetic leakage after mitral valve reoperation due to recurrent endocarditis. Catheter Cardiovasc Interv. 2009; 73:838–841.
3. Pate GE, Al Zubaidi A, Chandavimol M, Thompson CR, Munt BI, Webb JG. Percutaneous closure of prosthetic paravalvular leaks: case series and review. Catheter Cardiovasc Interv. 2006; 68:528–533.
4. Hourihan M, Perry SB, Mandell VS, et al. Transcatheter umbrella closure of valvular and paravalvular leaks. J Am Coll Cardiol. 1992; 20:1371–1377.
5. Eisenhauer AC, Piemonte TC, Watson PS. Closure of prosthetic paravalvular mitral regurgitation with the Gianturco-Grifka vascular occlusion device. Catheter Cardiovasc Interv. 2001; 54:234–238.
6. Webb JG, Pate GE, Munt BI. Percutaneous closure of an aortic prosthetic paravalvular leak with an Amplatzer duct occluder. Catheter Cardiovasc Interv. 2005; 65:69–72.
7. Binder RK, Webb JG. Percutaneous mitral and aortic paravalvular leak repair: indications, current application, and future directions. Curr Cardiol Rep. 2013; 15:342.
8. Nietlispach F, Johnson M, Moss RR, et al. Transcatheter closure of paravalvular defects using a purpose-specific occluder. JACC Cardiovasc Interv. 2010; 3:759–765.
9. Pate GE, Thompson CR, Munt BI, Webb JG. Techniques for percutaneous closure of prosthetic paravalvular leaks. Catheter Cardiovasc Interv. 2006; 67:158–166.
10. Cortés M, García E, García-Fernandez MA, Gomez JJ, Perez-David E, Fernández-Avilés F. Usefulness of transesophageal echocardiography in percutaneous transcatheter repairs of paravalvular mitral regurgitation. Am J Cardiol. 2008; 101:382–386.
11. Kort HW, Sharkey AM, Balzer DT. Novel use of the Amplatzer duct occluder to close perivalvar leak involving a prosthetic mitral valve. Catheter Cardiovasc Interv. 2004; 61:548–551.
12. Shapira Y, Hirsch R, Kornowski R, et al. Percutaneous closure of perivalvular leaks with Amplatzer occluders: feasibility, safety, and shortterm results. J Heart Valve Dis. 2007; 16:305–313.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download