Abstract
A 77-year-old female patient underwent aortic valve replacement (AVR) with concomitant septal myectomy and tricuspid annuloplasty. Her symptoms did not improve after a successful operation. Echocardiogram demonstrated the presence of an iatrogenic ventricular septal defect (VSD). It was muscular in location and not the usual AVR with membraneous type of VSD, suggesting a complication from the myectomy. Percutaneous closure of the VSD remained the only feasible option due to her poor overall medical status. A 14-mm Amplazter VSD occluder was deployed successfully, by means of the trans-septal technique. She has improved very well postoperatively.
Approximately 10% of patients with severe aortic valve stenosis (AS) have asymmetric basal septal hypertrophy (ABSH), relative to the posterior left ventricle (LV) free wall.1) The presence of ABSH and the need for myectomy may present a dilemma for surgeons performing aortic valve replacement (AVR). Without a concomitant myectomy, the gradient across with the left ventricular outflow tract (LVOT) can persist in patients with unresected ABSH.2) Many authors have reported that concomitant myectomy should be considered for patients with a preoperative diagnosis of ABSH, even without a demonstrated significant obstruction.3) Although it is known that concomitant myectomy with AVR is relatively safe and effective, complications beyond surgeons' control, such as iatrogenic ventricular septal defect (VSD) and atrioventricular block, may be developed. The presence of a hemodynamically significant VSD with left to right shunt may result in a left ventricular volume overload, and a prompt closure is necessary to prevent complications such as ventricular dysfunction, arrhythmias, aortic regurgitation and pulmonary hypertension.4)5) We describe a successful percutaneous closure of an iatrogenic VSD, following concomitant septal myectomy at the time of AVR.
A 77-year-old female was diagnosed with severe AS and ABSH. On echocardiography, the patient showed a restriction of aortic cusp movement, raised trans-aortic peak instantaneous velocity of Vmax (5.93 m/s), mean pressure gradient of 80.49 mm Hg, peak pressure gradient of 140.56 mm Hg and a narrowed effective orifice area of 0.64 cm2. She also manifested moderate tricuspid regurgitation (TR) with a dilated tricuspid annulus and an elevated right ventricular systolic pressure (RVSP) of 56 mm Hg. Based on indications for surgery in AS, she underwent AVR using a 19-mm Carpentier-Edward valve (CE valve, Edwards Lifesciences LLC, Irvine, CA, USA) with concomitant septal myectomy and tricuspid annuloplasty (TAP), using a 28-mm Edwards MC3 (Edwards Lifesciences LLC, Irvine, CA, USA).
After a successful operation, the patient complained of worsening dyspnea. On physical examination, a new continuous pansystolic murmur was noted. Echocardiogram demonstrated an abnormal high-velocity jet between the LVOT and right ventricle (RV) with a raised velocity of 4.78 m/s and peak pressure gradient of 91.33 mm Hg, suggesting the presence of a possible iatrogenic VSD (Fig. 1). The VSD was muscular in location, suggesting of complication by myectomy, not AVR in which it is usually present with membraneous type of VSD. The diameter of the VSD was about 12 mm, and the distance from the tissue aortic valve was about 1.5-2 cm. There was also an RV volume overload with moderate TR and an elevated RVSP of 61 mm Hg, despite the TAP. Medical treatments to maintain an optimal volume status was not effective, and the overall medical condition was poor. Therefore, a closure of the iatrogenic VSD with percutaneous intervention was decided, as it was felt to be hemodynamically significant.
At the right heart catheterization with the blood oximetry series to assess the degree of intra-ventricular shunting, there were remarkably elevated RV and pulmonary artery (PA) filling pressures, with an unidirectional left to right shunt of 2 : 1 (Qp/Qs=2).
On the 5th postoperative day, percutaneous closure of the VSD was attempted. It was decided that an attempt would be made to cross the VSD first from the RV (trans-septal technique). Left femoral artery and right femoral vein were punctured with 7 Fr sheaths. An LV angiogram showed a significant flow into RV cavity. Crossing of the VSD was made from the right side, using a 0.035-inch hydrophilic guidewire (angled straight tip, Terumo Medical, Tokyo, Japan) supported by a 5 Fr multipurpose catheter (Infiniti; Cordis, Miami, FL, USA), which was advanced into the LV apex. Then, the guidewire was replaced by a 0.035-inch extra-stiff guide wire (260-cm length, Amplatz Extra-Stiff, Cook, Bloomington, IN, USA). Then, a 14-mm Amplazter VSD occluder (AGA Medical Corporation, Golden Valley, MN, USA) attached to a delivery cable was advanced through the defect. The LV disc of the Amplazter VSD occluder was deployed first in the LV, and released the RV disc of the device in the RV by rotating the delivery cable in a counterclockwise direction (Fig. 2). An LV angiogram was performed confirming a minimal residual VSD flow, and intraoperative transesophageal echocardiogram demonstrated an excellent positioning of the device without shunting or any interference with aortic valve functions. At postprocedural catheterization, the PA pressure and saturation were decreased dramatically, from 50/15 mm Hg (mean 26 mm Hg), 91%, to 31/17 mm Hg (mean 23 mm Hg), 75%. Postprocedural echocardiogram showed a minimal shunt between the LVOT and RV without LVOT obstruction (Fig. 3).
Iatrogenic VSD following concomitant septal myectomy with AVR is not a common complication, and may warrant closure. A potential mechanism leading to iatrogenic VSD following septal myectomy may be related to myocardial tissue necrosis by obstruction of the septal perforator arteries, with the incisions or excisions made too deep.6)7) Repeat sternotomy for surgical closure of the VSD seems undesirable, and percutaneous VSD closure presents an attractive alternative. The success in eliminating hemodynamically-significant shunts produce stabilization and improvement in the signs and symptoms for which the percutaneous VSD closure is undertaken. Some reports of patients who have undergone percutaneous VSD closure following septal myectomy have been published in literature, with reasonable results and limited complications.8)9) Percutaneous VSD closure with trans-aortic approach would be unsafe in patients with AVR, because a bulky VSD device might get entrapped in the artificial aortic valve. Therefore, transseptal approach from the RV side would be better than trans-aortic approach in such patients. The challenge of VSD closure by trans-septal approach lies in crossing the guidewire across the VSD and the safe positioning of VSD device in the LV. More than necessary manipulations of a guidewire in the ventricles-RV or LV should be avoided to minimize the risks of ventricular arrhythmia or free-wall perforation.
In summary, percutaneous VSD closure may appear to ameliorate the pathologic changes that flow from hemodynamically-significant left to right shunting, easily, safely and effectively. We believe that through this case report, we may strengthen the use of percutaneous VSD closure in the cases of iatrogenic VSD following concomitant septal myectomy with AVR.
Figures and Tables
Fig. 1
Iatrogenic VSD assessment by echocardiography. A: parasternal long-axis view shows a muscular VSD (arrow), suggesting of a complication by myectomy, not AVR in which it is usually present with membraneous type of VSD. B: color Doppler imaging depicts a high-velocity jet between the left ventricle and right ventricle. C: the velocity and peak pressure gradient across the defect are measured at 4.78 m/s and 91.33 mm Hg, respectively. VSD: ventricular septal defect, AVR: aortic valve replacement, RV: right atrium, LV: left ventricle, Ao: aorta.
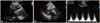
Fig. 2
Placement of Percutaneous Amplatzer Muscular ventricular septal defect (VSD) occluder. A: a 0.035 inch extra-stiff guide wire (arrow) is advanced from the femoral vein through the defect, and into the left ventricle (LV) apex. B: the diameter of Amplazter VSD occluder is measured using an Amplazter sizing balloon II (arrow). C: the LV disc of the Amplazter VSD occluder (arrow) is deployed first in the LV. D: it has released the right ventricle (RV) disc of the device (arrow) in the RV by rotating the delivery cable in a counterclockwise direction.
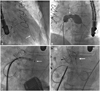
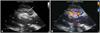
Fig. 3
Post-procedural echocardiography. A: parasternal long axis view shows the appropriate positioning of the Amplazter ventricular septal defect occluder. The device is seen as a dense crescent-like structure (arrow), at the left side of the interventricular septum. The left ventricular outflow tract is not obstructed. B: color Doppler imaging shows near-elimination of the left to right shunt after device delivery. RV: right ventricle, LV: left ventricle, LA: left atrium, Ao: aorta.
References
1. Panza JA, Maron BJ. Valvular aortic stenosis and asymmetric septal hypertrophy: diagnostic considerations and clinical and therapeutic implications. Eur Heart J. 1988; 9:Suppl E. 71–76.
2. Kayalar N, Schaff HV, Daly RC, Dearani JA, Park SJ. Concomitant septal myectomy at the time of aortic valve replacement for severe aortic stenosis. Ann Thorac Surg. 2010; 89:459–464.
3. Hess OM, Schneider J, Turina M, Carroll JD, Rothlin M, Krayenbuehl HP. Asymmetric septal hypertrophy in patients with aortic stenosis: an adaptive mechanism or a coexistence of hypertrophic cardiomyopathy? J Am Coll Cardiol. 1983; 1:783–789.
4. Carminati M, Butera G, Chessa M, et al. Transcatheter closure of congenital ventricular septal defects: results of the European Registry. Eur Heart J. 2007; 28:2361–2368.
5. Holzer R, de Giovanni J, Walsh KP, et al. Transcatheter closure of perimembranous ventricular septal defects using the amplatzer membranous VSD occluder: immediate and midterm results of an international registry. Catheter Cardiovasc Interv. 2006; 68:620–628.
6. Minakata K, Dearani JA, O'Leary PW, Danielson GK. Septal myectomy for obstructive hypertrophic cardiomyopathy in pediatric patients: early and late results. Ann Thorac Surg. 2005; 80:1424–1429. discussion 1429-30.
7. Valeti US, Nishimura RA, Holmes DR, et al. Comparison of surgical septal myectomy and alcohol septal ablation with cardiac magnetic resonance imaging in patients with hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol. 2007; 49:350–357.
8. Kilicgedik A, Karabay CY, Aung SM, et al. A successful percutaneous closure of ventricular septal defect following septal myectomy in patients with hypertrophic obstructive cardiomyopathy. Perfusion. 2012; 27:253–255.
9. Spies C, Ujivari F, Schraeder R. Transcatheter closure of a ventricular septal defect following myectomy for hypertrophic obstructive cardiomyopathy. Cardiology. 2009; 112:31–34.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download