Abstract
A 71-year-old woman who had suffered from pulmonary thromboembolism with deep vein thrombosis for 12 years presented the hospital with a huge thoracic aortic aneurysm. During thoracic endovascular therapy, she had a sudden coronary artery occlusion without having organized stenosis or plaque rupture even under the dual antiplatelet treatment and heparinization. She turned out to be having a protein S deficiency. A procedure related thrombotic adverse event in patient with protein S deficiency is very rare, so we report a case with literature review.
With a lower complication rate and satisfactory results, thoracic endovascular therapy has become a suitable alternative treatment for thoracic aneurysm.1) However, complications of the procedure including stroke, spinal cord ischemia or myocardial infarction are still in existence, leading problematic sequelae.2) A protein S deficiency is a well known genetic tendency to the venous thromboembolism.3) It can also be the risk factors for arterial occlusive diseases including coronary artery occlusion.4)5) However, it is not ge-nerally concerned as a procedural risk factor for endovascular treat-ment, thus special preparation for this thrombophilic disorder is not usually achieved during the peri-procedural period. In this report, we describe a rare case of peri-procedural thrombotic occlusion of coronary artery during thoracic endovascular aortic repair (TEVAR) in protein S deficiency and a literature review.
A 71 year-old woman who did not have a history of smoking was admitted to our hospital for treatment of the thoracic aortic aneurysm. She underwent a radical hysterectomy with bilateral salpingo oophorectomy in 1996 due to stage Ib of cervical cancer. She has also had a medical history of anticoagulation due to the pulmonary thromboembolism with deep vein thrombosis in both legs since 2001. However, she had quit her medication for 1 month without doctor's permission prior to this admission. There was a 11 cm diameter of thoracic aortic aneurysm containing mural thrombus with contrast leakage at the descending aorta on pre-procedural CT (Fig. 1A and B). A chronic eccentric embolism on the left pulmonary artery was also found in the CT angiography (Fig. 1C). An echocardiography indicated normal systolic function of the left ventricle without any intra-cardiac thrombi. On laboratory study, her anti-phospholipid IgG antibody and the tumor markers of CEA and CA19-9 were within the normal value. A protein C antigen level of the patient was also normal, but the protein S antigen was lower at 44.2% (normal range; 60-150%). Since the size of the patient's aneurysm was very large despite the absence of any symptoms, there might have been a risk of aortic rupture. Therefore, we planned TEVAR for her aneurysm.
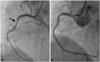
A 300 mg of aspirin and the 600 mg of clopidogrel were administrated orally on the day of the procedure. A Rt. common femoral artery (CFA) was prepared for the 22 Fr large sheath insertion by preclose technique,6) and the 7 Fr sheath was inserted after puncture via Lt. CFA. Then, 5000 IU (100 IU/kg) of heparin was administrated intravenously for endovascular treatment. After the marker pig tail catheter was placed at the aortic arch via Lt. CFA, a 5 Fr multipurpose catheter with 0.035 coating guide wire (J tip, Terumo, Tokyo, Japan) was located at the ascending artery via Rt. CFA. Then, the lunderquist extra-stiff guide wire (Cook, Inc., Bloomington, IL, USA) was inserted into the multipurpose catheter after coating guide wire was removed from it. However, during the wire exchanging procedure, the patient complained about a severe chest pain. The ECG of the monitor showed ST elevation of avF limb leads, and the bradycardia with hypotension was documented (Fig. 2B). A prompt coronary angiography was performed and it revealed a large amount of mobile thrombi with Thrombolysis in Myocardial Infarction 1 coronary blood flow in right coronary artery (Fig. 3A). However, neither coronary artery stenosis nor the plaque rupture was found in the coronary artery (Fig. 3B) after the thrombus aspiration (Eliminate aspiration catheter, Terumo, Tokyo, Japan). After achieving the patient's stabilization, 30 mm by 150 mm of thoracic stent graft was first deployed at the descending aorta. Then, a 34 mm by 200 mm of the stent graft was deployed at the aortic arch just distally to the left subclavian artery (Seal thoracic stent graft, S&G Inc, Seongnam, Korea) (Fig. 4A). A creatine kinase-MB was increased 1.9 to 5.4 ng/mL 6 hours after the procedure without increase of troponin-T. Post-procedural CT angiography showed a complete coverage of aneurysm without any leakage (Fig. 4B).
Thoracic endovascular aortic repair for descending thoracic aortic aneurysm is becoming the standard alternative therapy because of good applicability of endogarfting. As it is remarkably less morbid than surgical treatment, it leads to more expanded inclusion for the patient who has many medical conditions.2) Yet, still the peri-procedural complications after TEVAR "including" myocardial infarction, major stroke and the spinal cord infarction "are" the considerable demerits making the patient permanent sequalea.
Having an aortic aneurysm can be a risk factor for the peri-operative adverse outcome in itself because most aortic aneurysms are resulted from atherosclerotic degeneration. Therefore, it seems to be firmly associated with other types of atherosclerosis which are manifested by the coronary or peripheral arterial disease.7) For this reason, the risk of peri-procedural or peri-operative myocardial infarction rate was reported as high as 6% during aneurysm repair.8) It is not surprising that patients with more comorbidities had a significantly higher rate of major adverse event than those who did not have the risk factors.9)
However, protein S deficiency was not generally considered as a risk factor for intervention. Inherited thrombophilia including anticoagulant protein deficiency is known as a genetic tendency to venous thromboembolism. An anticoagulant protein S deficiency were reported in 7.3% of the patients who had deep vein thrombosis,3) and 74% and 38% of the patients with protein S deficiency had sustained deep venous thrombosis or pulmonary embolism, respectively, which is similarly to the patients in the current report.10) Unlike venous thrombosis, there is a lack of evidence for proving a risk factor for the development of arterial thrombosis, only a few studies of protein S deficiency demonstrated a high prevalence of arterial occlusive disease, ischemic stroke or cerebral ischemia.4)5)11) It is interesting that while several reports indicated coronary thrombosis in patients with protein S deficiency, their coronary arteries did not have organic stenosis or evidence of plaque rupture.12)13)14) Although the patient in current report was medicated antiplatelet agents and administered heparin before the procedure, the current phenomenon is likely to arise from embolic event if considering the pathophysiology of protein S deficiency. The source of thrombus might be from the coated wire surface and then could be dislodged while catheters exchange procedure. Thus, checking the activated coagulation time is mandatory before initiation of procedure and more cautious peri-procedural device handling under heparinized fluid is required for high risk patients having thrombophilia.
Another important consideration for the anticoagulant protein deficiency is the timing of testing in the laboratory evaluation since erroneous diagnoses can be made by the clinical situations as acute thrombosis, serious illness or anticoagulant therapy.15) However, the patient in the current report had good physical activity without severe illness at the time of admission and had discontinued warfarin arbitrarily for 1 month prior to the admission. The sample was taken at the time of the admission when the coumadization was not initiated.
We experienced a rare thrombotic coronary occlusion during the TEVAR and overcame the situation with immediate response, which yielded/produced favorable results preventing myocardial infarction. We prescribed warfarin and dual antiplatelet including the aspirin and clopidogrel, then continued the regimen for 6 months without any clinical events. Current case report contributes to a better understanding of the thrombotic complications during endovascular procedure and also thrombophilic diseases including protein S deficiency to the arterial occlusive adverse events. Hence, extra caution is required when performing endovascular treatment in patients with a thrombophlic disease.
Figures and Tables
Fig. 1
Pre-procedural aorta CT of presented patient. A: axial CT image shows the huge aneurysm (11 cm of diameter) containing the thrombus. B: reconstructed CT image shows the huge aneurysm containing the mural thrombus and the contrast leakage is seen inside the aneurysm as well. C: axial CT image shows the organized thrombi at the left main pulmonary artery.
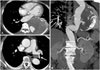
Fig. 2
Baseline and the event polygraphic records show the 3 limb leads ECG and the pulse pressure at the right common femoral artery sheath. A: baseline ECG shows 70 beats per minute of heart rate without significant ST height difference and blood pressure is approximately 110/60 mm Hg. B: an ECG at the time of chest pain indicates newly developed ST elevation at avF limb lead compared to the baseline ECG with 45 beats per minute of heart rate and the blood pressure is approximately 80/50 mm Hg. ECG: electrocardiography.

Fig. 3
Right coronary artery angiography before and after the thrombus aspiration. A: right coronary angiography shows the mobile multiple thrombi inside of the vessels and the TIMI grade 1 of blood flow. B: right coronary angiography after the thrombus aspiration shows no organic stenosis or remnant thrombi inside the vessel and the TIMI grade 4 of blood flow is restored. TIMI: Thrombolysis in Myocardial Infarction.
References
1. Walsh SR, Tang TY, Sadat U, et al. Endovascular stenting versus open surgery for thoracic aortic disease: systematic review and meta-analysis of perioperative results. J Vasc Surg. 2008; 47:1094–1098.
2. Desai ND, Burtch K, Moser W, et al. Long-term comparison of thoracic endovascular aortic repair (TEVAR) to open surgery for the treatment of thoracic aortic aneurysms. J Thorac Cardiovasc Surg. 2012; 144:604–609. discussion 609-11.
3. Mateo J, Oliver A, Borrell M, Sala N, Fontcuberta J. Laboratory evaluation and clinical characteristics of 2,132 consecutive unselected patients with venous thromboembolism--results of the Spanish Multicentric Study on Thrombophilia (EMET-Study). Thromb Haemost. 1997; 77:444–451.
4. Allaart CF, Aronson DC, Ruys T, et al. Hereditary protein S deficiency in young adults with arterial occlusive disease. Thromb Haemost. 1990; 64:206–210.
5. Douay X, Lucas C, Caron C, Goudemand J, Leys D. Antithrombin, protein C and protein S levels in 127 consecutive young adults with ischemic stroke. Acta Neurol Scand. 1998; 98:124–127.
6. Lee WA, Brown MP, Nelson PR, Huber TS. Total percutaneous access for endovascular aortic aneurysm repair ("Preclose" technique). J Vasc Surg. 2007; 45:1095–1101.
7. Sukhija R, Aronow WS, Yalamanchili K, Sinha N, Babu S. Prevalence of coronary artery disease, lower extremity peripheral arterial disease, and cerebrovascular disease in 110 men with an abdominal aortic aneurysm. Am J Cardiol. 2004; 94:1358–1359.
8. Bub GL, Greenberg RK, Mastracci TM, et al. Perioperative cardiac events in endovascular repair of complex aortic aneurysms and association with preoperative studies. J Vasc Surg. 2011; 53:21–27.e1-2.
9. Wang GJ, Fairman RM, Jackson BM, Szeto WY, Pochettino A, Woo EY. The outcome of thoracic endovascular aortic repair (TEVAR) in patients with renal insufficiency. J Vasc Surg. 2009; 49:42–46.
10. Engesser L, Broekmans AW, Briët E, Brommer EJ, Bertina RM. Hereditary protein S deficiency: clinical manifestations. Ann Intern Med. 1987; 106:677–682.
11. Munts AG, van Genderen PJ, Dippel DW, van Kooten F, Koudstaal PJ. Coagulation disorders in young adults with acute cerebral ischaemia. J Neurol. 1998; 245:21–25.
12. Beattie S, Norton M, Doll D. Coronary thrombosis associated with inherited protein S deficiency: a case report. Heart Lung. 1997; 26:76–79.
13. Carrié D, Béard T, Sié P, Boudjemaa B, Delay M, Bernadet P. [Simultaneous thrombosis of the left anterior interventricular and right coronary arteries in a 27 year-old patient with protein S deficiency]. Arch Mal Coeur Vaiss. 1993; 86:921–924.
14. Manzar KJ, Padder FA, Conrad AR, Freeman I, Jonas EA. Acute myocardial infarction with normal coronary artery: a case report and review of literature. Am J Med Sci. 1997; 314:342–345.
15. Schwarz HP, Fischer M, Hopmeier P, Batard MA, Griffin JH. Plasma protein S deficiency in familial thrombotic disease. Blood. 1984; 64:1297–1300.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download