Abstract
Background and Objectives
We investigated the effects of commonly used contrast media (CM) on myocardial ischemia-reperfusion injury in isolated rat hearts.
Subjects and Methods
Isolated rat hearts were subjected to 30 minutes of regional ischemia and 2 hours of reperfusion. The following CM (1 mL/1 L Krebs-Henseleit buffer) were randomly perfused for 15 minutes beginning 5 minutes before reperfusion and ending 10 minutes after reperfusion: iohexol (n=8), iopromide (n=8), ioversol (n=8), iomeprol (n=8), iopamidol (n=7), ioxaglate (n=8), and iodixanol (n=7). The effects of a direct bolus injection of undiluted iohexol, iopromide, or ioxaglate (each n=6) via the aortic root immediately prior to reperfusion were also evaluated. The area of necrosis, expressed as the percentage of the area at risk (AN/AR), and cardiodynamic variables were measured.
Results
The AN/AR of the control and experimental groups in the order described in methods was 33.7±6.4%, 30.3±7.4%, 34.7±12.6%, 29.2±10.2%, 20.9±7.6%, 22.6±8.7%, 18.8±7.9%, and 19.9±11.4%, respectively. Groups that received iomeprol and ioxaglate exhibited significantly decreased AN/AR values compared to those of control hearts (p=0.042 and p=0.013). No significant differences in the AN/AR were observed between control hearts and the groups injected with a single bolus of CM. No significant hemodynamic changes were noted after reperfusion among the groups.
Interventional cardiology commonly uses contrast media (CM), but the effects of commonly used CM on reperfusion injury have not been thoroughly evaluated. Many studies have compared the effects of CM on various organs, and the side effects of CM on kidneys have been studied. The risk of contrast-induced nephropathy increases if a CM is dimeric, has a higher osmolality, or if the total volume of CM is greater.1)2)3)4) The lack of experimental and clinical studies is quite surprising considering that the myocardium is supposed to be exposed to a CM immediately after coronary reperfusion. The purpose of this study was to investigate the direct effects of commonly used CM on ischemic hearts undergoing reperfusion and the possible mechanisms of these effects.
The isolated rat hearts randomly received a monomer or dimer CM from one of the following three groups: 1) iohexol, a non-ionic and low-osmolality monomer CM (Omnipaque 350®, GE Healthcare, Piscataway, NJ, USA), iopromide (Ultravist 370®, Bayer Healthcare, Seoul, Korea), ioversol (Optiray 350®, Tyco Healthcare, Tokyo, Japan), iomeprol (Iomeron 350®, Bracco Imaging Korea, Seoul, Korea), or iopamidol (Pamiray 370®, Dong Kook Pharmacy, Seoul, Korea); 2) an ionic and low-osmolality dimer CM ioxaglate (Hexabrix 320®, Guerbet, Villepinte, France) or 3) a non-ionic iso-osmolality dimer CM iodixanol (Vispaque 320®, GE Healthcare, Piscataway, NJ, USA). The physiochemical properties of the CM are shown in Table 1 and Fig. 1. The osmolality and viscosity of Krebs-Henseleit (KH) buffer was 290 mOsm/kg H2O and 6.9 mPa · s at 37℃, and were cited previously.5)6)
The experimental procedures and protocols used in this study were reviewed and approved by our Institutional Animal Care and Use Committee. Male Sprague-Dawley rats (KOATECH Co., Cheong-won, Korea), weighing 280-330 g, were used. The rats received 50 mg/kg pentobarbital sodium (Entobar®, Hanlim Pharmacy, Yongin, Korea) and 300 IU heparin intraperitoneally. The hearts were isolated and perfused with modified KH buffer containing (all in mM) 118.5 NaCl, 4.7 KCl, 1.2 MgSO4, 1.8 CaCl2, 24.8 NaHCO3, 1.2 KH2PO4, and 10 glucose. All hearts were perfused within 30-40 seconds after excision and were allowed to stabilize for at least 20 minutes. Regional ischemia was induced by making a snare around the major trunk of the left coronary artery (LCA) or its prominent branches between the left atrial appendage and the right ventricular outflow tract. Reperfusion was initiated by releasing the ends of the snare. All hearts were subjected to 30 minutes of regional ischemia and 2 hours of reperfusion. Control (CON) hearts (n=7) received no intervention either before or after LCA occlusion. The CM were given by two concentration models.
The CM were dissolved in KH buffer (1 mL/1 L KH buffer) on the day of the experiment and perfused for 15 minutes starting 5 minutes before reperfusion and ending 10 minutes after reperfusion. The 1:1000 dilution was chosen to investigate the pharmacological effects of CM and minimize the effects of physiochemical properties such as osmolality and viscosity of each CM.
Additional rat hearts (each n=6) received a single bolus of 3 mL pure iohexol (Iohexol-S group), iopromide (iopromide-S group), or ioxaglate (ioxaglate-S group) via the aortic root immediately before reperfusion to complement for the loss of physiochemical properties by diluting the CM.
Myocardial contractility was assessed by left ventricular developed pressure (LVDP). An air-bubble-free, KH buffer-filled latex balloon was inserted into the left ventricle (LV) of the isolated hearts through the left atrial appendage. Balloon volume was adjusted with the BIOPAC system (BIOPAC Systems Inc., Goleta, CA, USA) to provide and sustain a left ventricular end-diastolic pressure (LVEDP) of 5-10 mm Hg from the beginning of the experiment. The LVDP was calculated as the difference between the left ventricular systolic pressure and the LVEDP. +dP/dtmax was obtained using the analytical software (BSL v3.7.3, BIOPAC Systems Inc., Goleta, CA, USA).
At the end of experiment, the area at ischemic risk (AR) and the necrotic area (AN) were demarcated with diluted fluorescent polymer microspheres (Duke Scientific Corp., Palo Alto, CA, USA) and 2,3, 5-triphenyltetrazolium chloride (Sigma-Aldrich Chemical, St. Louis, MO, USA) stain. The AR and AN areas in the LV were quantified with UTHSCSA Image Tool ver. 3.0 and converted into volume by multiplying the areas by slice thickness (2 mm) with a rat heart slicer (Zivic Instruments, Pittsburgh, PA, USA). AN volume is expressed as a percentage of AR volume. All morphometric measurements were performed blind.
No significant differences were observed in baseline LV mass, AR, AN, or AR/LV (%) between the CON and experimental groups. AR/LV ranged from 49.8% to 58.7% (p>0.05) (Table 2), suggesting that change in infarct volume was not related to the degree of ischemic volume. As shzown in Table 2, AN/AR in CON hearts was 33.7±6.4%. Representative imaging data obtained during the infarct size measurements are shown in Fig. 2.
Among hearts that received monomer CM, iomeprol (20.9± 7.6%) significantly decreased the AN/AR value compared to that in CON hearts (p=0.042). The ionic dimer ioxaglate (18.8±7.9%) also significantly decreased AN/AR compared to that in CON hearts (p= 0.013). A slight tendency to decrease infarct size was noted in the other CM groups, except the iopromide group (p>0.05) (Fig. 3, Table 2).
AN/AR values for the iopromide-S, ioxaglate-S, and iohexol-S groups were 36.5±7.8%, 27.9±8.0%, and 27.3±5.7%, respectively. This protocol mimicked the actual primary percutaneous coronary intervention by shooting bolus CM via the aortic root. No significant difference in infarct size was observed between CON hearts (33.7±6.4%) and the experimental groups (Fig. 4, Table 2).
Seventy-nine rat hearts were used for this experiment, and ventricular fibrillation (VF) occurred in 37 hearts (4/7 in CON, 5/8 in iohexol, 4/8 in iopromide, 4/8 in ioversol, 5/8 in iomeprol, 3/7 in iopamidol, 5/8 in ioxaglate, 2/7 in iodixanol 2/7, 3/6 in iohexol-S, 3/6 in iopromide-S, and 2/6 in ioxaglate-S) during early reperfusion. A statistical analysis was not performed for the incidence of VF because of the small sample size. No significant differences were observed in baseline heart rate (HR), LVDP, or +dP/dtmax among the groups (Table 3). Iodixanol preserved HR after reperfusion (p=0.041 vs. CON). However, no significant differences were observed among the groups for the other hemodynamic parameters after reperfusion.
The harmful effects of CM on contrast-induced acute kidney injury (CIAKI) were described in terms of three factors: 1) direct toxicity of iodine, 2) higher osmolality, and 3) reduced flow rate due to viscosity.7)8) The higher the osmolality and viscosity of a CM, the greater the risk of CIAKI.7)9) Several interesting studies have been conducted to determine whether viscosity and osmolality, which are very important factors for CIAKI, also negatively affect ischemia-reperfusion injury.
Falck et al.10) reported a significant decrease in infarct size in isolated rat hearts that received repetitive injections of ioxaglate, iodixanol, or iohexol compared to that in the CON group. The authors explained the decrease in infarct size in conjunction with the "preconditioning" effect of osmolality. The end-products of anaerobic metabolism accumulate in ischemic myocardium; thus, increasing osmotic load in the intracellular and interstitial spaces. Osmotically active molecules in the extracellular space are rapidly washed out after reperfusion, forming an osmotic gradient between the intra-and extracellular environments and causing cell swelling, resulting in increased cell fragility and cell death.11) This phenomenon is called hyperosmotic stress. Several studies have indicated that hearts pretreated with hyperosmolar agents are resistant to hypoxia.12)13) In those experiments, various hyperosmolar agents, mostly -600 mM buffers, produced effective preconditioning results. These findings are somewhat different from the effect of CM, which is suggested to be deleterious in CIAKI.
Data on the effects of CM viscosity on ischemia are conflicting. Hyperviscosity understandably increases vascular resistance, which decreases blood flow, resulting in hypoxia, according to the Hagen-Poiseuille equation. Some clinical evidence suggests possible deleterious effects of hyperviscosity on coronary reperfusion.14)15) However, hyperviscosity increases the transit time of blood passing th-rough capillaries and venules; thus, increasing oxygen extraction and favoring oxygenation of ischemic tissue. This also increases shear stress, which recruits endothelial protective factors, such as nitric oxide.16)
The effects of CM on coronary reperfusion after ischemia are expected to be harmful based on CIAKI. However, according to some studies, neither CM nor their physiochemical properties, which have been suggested to be harmful in CIAKI, have been demonstrated to be deleterious during ischemia reperfusion of isolated rat hearts. None of the CM groups in this study showed decreased infarct size compared with that in the CON. We attempted to determine some of the possible effects of CM physiochemical properties, but no relationship among osmolality, viscosity, or infarction size was observed from the results of Protocol 2 (Supplementary Fig. 1 in the online-only Data Supplement).
Several adverse effects of various concentrations of iomeprol on the human cardiovascular system were reported in 1993, including blood pressure fall/hypotension, bradycardia, angina pectoris, and shock.17) In contrast, another study investigated the effects of non-ionic iodinated CM (iomeprol-350, iodixanol-320) on hemodynamics of the human heart by intra-cardiac or intra-arterial injection but the results showed minimal effects of the CM on HR and LV pressure.18) The effect of CM on infarct size has not been reported in a human study. This may be because estimating the effect of CM on ischemia-reperfusion is limited when designing a study due to various confounding factors, such as platelets, drugs, ischemic time, or difficulties measuring infarct size.
Several limitations in this study should be discussed. First, we evaluated the effect of CM on reperfusion using the Langendorff model, in which the hemorheological factors of whole blood were excluded. The viscosity of whole blood and the interaction of the CM with the platelet system could play a role reducing infarct size.13) As shown in cases of CIAKI, laboratory data may not directly correspond to clinical results.19)20)21) Second, other types of interactions among physiochemical properties, such as electric charges, were not controlled. In addition, the sample sizes were small. Infarct sizes tended to decrease in all groups except in the iopromide group.
Contrast media are necessary in interventional cardiology. In particular, CM are the very first materials that reach the myocardium after reperfusion, but their effects have scarcely been reported. The effects of CM on ischemia reperfusion in our study were not deleterious, and better effects were noted in some groups. Further studies are needed to identify the mechanisms.
Figures and Tables
Fig. 1
Physiochemical properties of the contrast media (CM), particularly focusing on osmolality and viscosity. Note that ioxaglate 320 and iodixanol 320 have similar viscosity and osmolality.

Fig. 2
Representative images obtained during measurements. The midportion of the left ventricle of a control heart (A) and a diluted ioxaglateperfused heart (B) after triphenyltetrazolium chloride (TTC) staining are shown. Intact myocardium can be discriminated by the fluorescent area under UV light. The other side of the dotted line is the area at risk (AR) (C). The area of necrosis (AN) is identified as the region not stained by TTC under natural light (D). UV: ultraviolet.
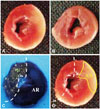
Fig. 3
Area of necrosis (AN) as a percentage of the area at risk (AR) in protocol 1. Contrast media (CM) was perfused continuously from 5 minutes before reperfusion to 10 minutes after reperfusion. Shaded box indicates the lower viscosity group. Values are means±standard deviations *p<0.05 vs. CON. CON: untreated control hearts, LOCM: low-osmolality CM, IOCM: iso-osmolality CM.

Fig. 4
Area of necrosis (AN) as a percentage of the area at risk (AR) in protocol 2. Iohexol-S, iopromide-S, and ioxaglate-S indicate single bolus injections of iohexol, iopromide, and ioxaglate, respectively. Values are means± standard deviations. No significant differences in AN/AR were observed between the CON and experimental groups. CON: untreated control hearts.
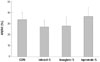
Table 1
Physiochemical properties of the contrast media

Table 2
Morphometric data

Table 3
Hemodynamic data

Values are means±standard deviations. Iohexol-S, iopromide-S, and ioxaglate-S indicate single bolus injection of iohexol, iopromide, and ioxaglate, respectively *p<0.05 vs. CON. CON: untreated control hearts, HR: heart rate, LVDP: left ventricular developed pressure, +dP/dtmax: velocity of left ventricular contraction
References
1. Heinrich MC, Kuhlmann MK, Grgic A, Heckmann M, Kramann B, Uder M. Cytotoxic effects of ionic high-osmolar, nonionic monomeric, and nonionic iso-osmolar dimeric iodinated contrast media on renal tubular cells in vitro. Radiology. 2005; 235:843–849.
2. Aspelin P, Aubry P, Fransson SG, et al. Nephrotoxic effects in high-risk patients undergoing angiography. N Engl J Med. 2003; 348:491–499.
3. Romano G, Briguori C, Quintavalle C, et al. Contrast agents and renal cell apoptosis. Eur Heart J. 2008; 29:2569–2576.
4. Wessely R, Koppara T, Bradaric C, et al. Choice of contrast medium in patients with impaired renal function undergoing percutaneous coronary intervention. Circ Cardiovasc Interv. 2009; 2:430–437.
5. Oriyanhan W, Yamazaki K, Miwa S, Takaba K, Ikeda T, Komeda M. Taurine prevents myocardial ischemia/reperfusion-induced oxidative stress and apoptosis in prolonged hypothermic rat heart preservation. Heart Vessels. 2005; 20:278–285.
6. Gonzalez-Castillo C, Rubio R, Zenteno-Savin T. Coronary flow-induced inotropism is modulated by binding of dextrans to the endothelial luminal surface. Am J Physiol Heart Circ Physiol. 2003; 284:H1348–H1357.
7. Seeliger E, Sendeski M, Rihal CS, Persson PB. Contrast-induced kidney injury: mechanisms, risk factors, and prevention. Eur Heart J. 2012; 33:2007–2015.
8. Sendeski MM. Pathophysiology of renal tissue damage by iodinated contrast media. Clin Exp Pharmacol Physiol. 2011; 38:292–299.
9. Seeliger E, Becker K, Ladwig M, Wronski T, Persson PB, Flemming B. Up to 50-fold increase in urine viscosity with iso-osmolar contrast media in the rat. Radiology. 2010; 256:406–414.
10. Falck G, Bruvold M, Schjøtt J, Jynge P. Protective effects of repetitive injections of radiographic contrast media on the subsequent tolerance to ischemia in the isolated rat heart. Cardiovasc Intervent Radiol. 2000; 23:466–471.
11. Piper HM, García-Dorado D. Prime causes of rapid cardiomyocyte death during reperfusion. Ann Thorac Surg. 1999; 68:1913–1919.
12. Falck G, Schjott J, Jynge P. Hyperosmotic pretreatment reduces infarct size in the rat heart. Physiol Res. 1999; 48:331–340.
13. Pastukh V, Ricci C, Solodushko V, Mozaffari M, Schaffer SW. Contribution of the PI 3-kinase/Akt survival pathway toward osmotic preconditioning. Mol Cell Biochem. 2005; 269:59–67.
14. Cecchi E, Liotta AA, Gori AM, et al. Relationship between blood viscosity and infarct size in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Int J Cardiol. 2009; 134:189–194.
15. Wasilewski J, Turczyński B, Słowińska L, Kowalik V, Osadnik T, Poloński L. Haemorheological factors and myocardial reperfusion in patients with ST-elevation myocardial infarction undergoing primary coronary intervention. Kardiol Pol. 2007; 65:778–785. discussion 786-7.
16. Yalcin O, Ulker P, Yavuzer U, Meiselman HJ, Baskurt OK. Nitric oxide generation by endothelial cells exposed to shear stress in glass tubes perfused with red blood cell suspensions: role of aggregation. Am J Physiol Heart Circ Physiol. 2008; 294:H2098–H2105.
17. Schmid I, Didier D, Pfammatter T, et al. Effects of non-ionic iodinated contrast media on patient heart rate and pressures during intra-cardiac or intra-arterial injection. Int J Cardiol. 2007; 118:389–396.
18. Schmiedel E. Evaluation of the adverse effects of iomeprol. Eur J Radiol. 1994; 18:Suppl 1. S104–S108.
19. Reed M, Meier P, Tamhane UU, Welch KB, Moscucci M, Gurm HS. The relative renal safety of iodixanol compared with low-osmolar contrast media: a meta-analysis of randomized controlled trials. JACC Cardiovasc Interv. 2009; 2:645–654.
20. Heinrich MC, Häberle L, Müller V, Bautz W, Uder M. Nephrotoxicity of iso-osmolar iodixanol compared with nonionic low-osmolar contrast media: meta-analysis of randomized controlled trials. Radiology. 2009; 250:68–86.
21. From AM, Al Badarin FJ, McDonald FS, Bartholmai BJ, Cha SS, Rihal CS. Iodixanol versus low-osmolar contrast media for prevention of contrast induced nephropathy: meta-analysis of randomized, controlled trials. Circ Cardiovasc Interv. 2010; 3:351–358.
Supplementary Materials
The online-only Data Supplement is available with this article at http://dx.doi.org/10.4070/kcj.2014.44.6.423.
Supplementary Fig. 1
Area of necrosis (AN) as a percentage of the area at risk (AR) according to osmolality (mOsm/kg H2O) and viscosity (mPa · s at 37℃) of the contrast media (CM) in protocol 2. Osmolality and viscosity in the CON group are those of the KH buffer. Horizontal lines represent mean values of the results. No significant relationship was observed among osmolality, viscosity, and infarct size.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download