Abstract
Idiopathic thrombocytopenic purpura (ITP) is an autoimmune disorder with a low platelet count characterized by premature platelet destruction and suppression of platelet production mediated by autoantibodies, which may predispose to bleeding. Although the prevalence of coronary artery disease (CAD) in ITP seems to be rare, their co-occurrence is not unusual. Patients with ITP have increased risks for thrombosis and atherosclerosis associated with hemostatic factors, endothelial damage, and the negative effects of steroid and immunoglobulin therapies. Thus, the coexistence of ITP and CAD presents complex problems requiring a balance between hemorrhagic risk and prevention of thrombosis. Here, the authors present two patients with ITP, who were revascularized in different ways for CAD. Although the optimal management of thrombocytopenic patients with CAD is uncertain, individualized treatment modalities can be useful in patients with ITP and CAD.
Idiopathic thrombocytopenic purpura (ITP) is an autoimmune disorder with a low platelet count characterized by premature platelet destruction and suppression of platelet production mediated by autoantibodies, which may predispose to bleeding.1) Since severe bleeding is uncommon when the platelet count exceeds 30000/uL, treatment is usually indicated when a platelet count is less.2) Considering the crucial role of platelets in thrombotic events, the prevalence of coronary artery disease (CAD) in patients with ITP seems to be uncommon, especially when the platelet count is low. However, the co-occurrence of ITP and CAD is not very unusual. Patients with ITP have increased risks for thrombosis and atherosclerosis associated with larger and more adhesive platelets, direct endothelial damage,3) and negative effects of therapy with steroids4) or intravenous immunoglobulin (IVIG).5)
Since relatively few case reports have been reported, no recommendations have been issued regarding the management of thrombocytopenic patients with CAD. Moreover, considering the high bleeding risk, a careful balance must be achieved between the prevention of thrombosis and hemorrhagic risk.
We present two patients with ITP and CAD who were successfully treated using different therapeutic strategies.
A 65-year-old man was transferred to our emergency room (ER) with epigastric pain. One month previously, he had been diagnosed with ITP. The patient had taken oral steroids for ITP (prednisolone 55 mg/day), which had increased the platelet count from 8000 to 73000/uL. At ER admission, his platelet count was 80000/uL. Electrocardiography (ECG) showed ST segment elevation in leads V 1-3, which was consistent with an anterior wall acute myocardial infarction. Cardiac enzyme (troponin I) was elevated (7.61 ng/mL, normal range: 0.0-0.1 ng/mL). Blood coagulation tests showed a prothrombin time (PT) of 13.4 seconds {international normalized ratio (INR) 1.15} and an activated partial thromboplastin time (aPTT) of 35.1 seconds. Primary percutaneous coronary intervention (PCI) with loading dose clopidogrel 300 mg and aspirin (ASA) 500 mg was performed. Coronary angiography (CAG) revealed nearly total occlusion with the Thrombolysis in Myocardial Infarction (TIMI) grade 2 in the proximal left anterior descending coronary artery (LAD) (Fig. 1A) and a normal right coronary artery (RCA). After balloon angioplasty, one drug eluting stent (DES, 3.0×28 mm, Coroflex® please; B. Braun Melsungen AG, Germany) was implanted in the proximal LAD, and achieved an optimal angiographic result and TIMI grade 3 flow in the LAD (Fig. 1B). After removing the sheath introducer, careful manual compression of the femoral puncture site was performed and no bleeding complication occurred. ECG after PCI showed complete resolution of ST segment elevation without chest pain. Daily treatment with ASA 100 mg and Clopidogrel 75 mg was continued and no symptoms or bleeding complication were noted after discharge.
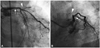
A 67-year-old man with a 10-year history of treatment resistant ITP was admitted with effort-induced chest pain (Canadian Cardiovascular Society class III). Ten years previously, a splenectomy was performed but it was ineffective in increasing platelet count. On admission, his platelet count was 12000/uL. Cardiac enzymes were not elevated. Blood coagulation tests showed a PT of 11.2 seconds (INR 1.04) and an aPTT of 30.2 seconds. ECG showed sinus bradycardia without ST segment or T wave change. Concerning about bleeding due to a low platelet count and gastrointestinal disturbance, antiplatelet medication maintained on a single antiplatelet regimen (Clopidogrel 75 mg/day). On hospital stay, he experienced an event of epistaxis while his platelet count was 18000/uL. He was administered two consecutive courses of IVIG and subsequent platelet transfusion and intravenous (IV) steroid (methylprednisolone 500 mg). Platelet count increased to 127000/uL. CAG revealed 60% stenosis in the left main trunk, total occlusion of the proximal LAD (TIMI grade 0 distal flow) with collateral flow TIMI grade 2 from the RCA to the LAD, and 50% luminal stenosis in the middle RCA (Fig. 2). Left internal mammary artery (LIMA) was intact. After careful discussion with the patient, a coronary artery bypass graft (CABG) strategy was chosen. After sheath removal, the Perclose ProGlide® 6 F device (Abbott Vascular, USA) was applied at the femoral puncture site without bleeding complication. While preparing for CABG, the platelet count dropped again to 14000/uL. He was then transferred to the hematologic department to properly address the thrombocytopenia. After 2 days of IVIG, IV steroid, and platelet transfusion, the platelet count increased to 189000/uL. CABG was performed using off-pump techniques, and the LIMA was connected to the LAD. To minimize the operation time, a single graft to the LAD was made rather than multivessel grafting. Initial activated clotting time was 136 seconds, IV unfractionated heparin 6000 IU (100 IU/kg) was administered. The operation time was 200 minutes. The perioperative period was uneventful, without excessive bleeding. IV steroid was continued and platelet counts remained stable throughout the postoperative period. He was discharged on postoperative day 12 with clopidogrel and oral prednisolone medications. Platelet count on discharge was 94000/uL. The antiplatelet drug was continued and no symptoms or bleeding complications were subsequently noted.
We present two patients with ITP who received different revascularization treatments for CAD. Although the prevalence of CAD in patients with ITP seems to be rare, considering the crucial role of platelets, the coexistence of ITP and CAD is not unusual. If a patient with chronic thrombocytopenia has CAD, then the presence of known cardiovascular risk factors, such as a relevant family history, hypertension, diabetes mellitus, dyslipidemia, chronic kidney disease, and cigarette smoking should be considered. Bleeding tendency is one of the most important problems of ITP and CAD, especially in patients with DES, because of long-term dual antiplatelet therapy. Thus, the appropriate use of periprocedural support treatment to increase platelet numbers probably minimized bleeding.
In ITP, thrombocytopenia itself does not protect against thrombosis, and cases of acute coronary syndrome have been reported.3) Accordingly it appears that some factors other than platelet number are involved. Patients with ITP may be predisposed to coronary thrombosis because their platelets are larger and more adhesive to vascular surfaces.6) Furthermore, an antigenic mimicry between platelets and endothelial cells may also lead to endothelial damage by autoantibodies directed against platelet surface antigens.3) In addition, steroid and IVIG treatment may be related to an increased risk of CAD and thrombosis in ITP.4)5) Steroids induce metabolic changes as well as a hypercoagulable state, both of which may promote atherosclerosis.7) Infusion of IVIG may induce expansion of plasma volume with subsequent hypertensive reactions, increased oxygen demand, and cardiac decompensation,8) and also increase in plasma and blood viscosity.9) In case 1, steroid therapy appeared to play a role as a precipitating agent with another factor, such as hyperglycemia.
Percutaneous coronary intervention has been successfully performed in ITP despite markedly different platelets counts (range from 3000/uL10) to 200000/uL11)) without major bleeding. However, performance of PCI in a patient with ITP presents a particular problem that requires sufficient inhibition of platelet function to prevent stent thrombosis (ST), but not enough to cause bleeding. Because DES requires long-term maintenance with dual antiplatelet agents to prevent ST, bare metal stents (BMS) are generally implanted during PCI in order to use the dual antiplatelet therapy in as less time as possible. However, like our case 1, DES can be safely implanted in a background of ITP and CAD without any serious bleeding complications.11)12) Although Neskovic et al.13) proposed a management algorithm of BMS placement followed by lifelong ASA and short termed Clopidogrel, we believe that DES can be applied safely in this subset of patients if platelet number and bleeding tendency are careful monitored.
Regarding the vascular approach, a radial artery is preferred due to evidence of a lower risk of bleeding, as well as easy hemostatic compression, early ambulation, and patient comfort.10)12)14) However, a vascular approach should be decided on having considered platelet count, degree of emergency, characteristics of coronary lesions, and vascular integrity. As described for our patients, both transfemoral and transradial approaches were successfully used for CAG or PCI without any bleeding complication.15) In addition, arterial sealing and closing devices have been demonstrated to be effective at achieving immediate hemostasis after sheath removal and to allow early ambulation, reduce local complications, and provide patient comfort.16) Such hemostatic devices may be especially helpful in patients with a high risk of bleeding and with performing through transfemoral route.
Historically, CABG is generally preferred to PCI in these ITP patients because it produces better results for all types of lesion and facilitates the management of antiplatelet therapy after the procedure.17) Despite the low platelet count, CABG was successfully carried out with a moderate increase in bleeding risk compared to CABG in the general population. The risk of significant bleeding was higher in the ITP population, 12.5%, compared to 5.4% in the general population.18) Perioperative treatments, such as prophylactic splenectomy, steroid treatment, IVIG, and platelet transfusion, may reduce complication rates by increasing platelet counts. In particular, some authors have suggested preoperative IVIG as a treatment of choice,18) especially when a rapid increase in platelet count is needed (as occurred in case 2). However, as mentioned above, associations have been reported between CAD and perioperative management, such as IVIG, steroid, and platelet transfusion. Thus, decision making should be done cautiously when considering such therapies.
Case 2 underwent CABG using an off-pump technique and a LIMA conduit. An off-pump CABG technique may be a safer strategy, because it avoids the use of an extracorporeal circuit and, thus, minimizes platelet dysfunction.19) One of the benefits of using off-pump CABG rather than conventional CABG is the significant reduction in blood loss and the reduced need for transfusion of platelets and other blood products.20) Moreover, given the minimal bleeding rate, extending conduit use including internal mammary arteries appears appropriate.18)
We present two patients with ITP and CAD, who were treated differently, using PCI and CABG. Although the optimal management of thrombocytopenic patients with CAD remains uncertain, our experiences of the two described cases suggest that both coronary revascularization techniques can be a useful strategy in these patients, even when thrombocytopenia is severe. We conclude that various revascularization strategies are possible in ITP patients with CAD and that treatment should be individualized after taking into consideration initial diagnosis and patient variables, such as platelet count and bleeding tendency.
Figures and Tables
Fig. 1
Intracoronary stent implantation in patient 1, who had idiopathic thrombocytopenic purpura. A: coronary angiography in right anterior oblique view shows near total occlusion (arrow) with the Thrombolysis in Myocardial Infarction grade 2 in the proximal left anterior descending coronary artery. B: percutaneous coronary intervention with a drug eluting stent was performed successfully.
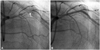
Fig. 2
Coronary angiography of patient 2 with idiopathic thrombocytopenic purpura. Coronary angiography in right inferior oblique view (A) and spider view (B) show 60% stenosis in the left main trunk (arrow), total occlusion of the proximal left anterior descending coronary artery with Thrombolysis in Myocardial Infarction grade 0 distal flow (arrowhead).
References
1. Cines DB, Bussel JB, Liebman HA, Luning Prak ET. The ITP syndrome: pathogenic and clinical diversity. Blood. 2009; 113:6511–6521.
2. Cines DB, Blanchette VS. Immune thrombocytopenic purpura. N Engl J Med. 2002; 346:995–1008.
3. Fruchter O, Blich M, Jacob G. Fatal acute myocardial infarction during severe thrombocytopenia in a patient with idiopathic thrombocytopenic purpura. Am J Med Sci. 2002; 323:279–280.
4. Paolini R, Zamboni S, Ramazzina E, Zampieri P, Cella G. Idiopathic thrombocytopenic purpura treated with steroid therapy does not prevent acute myocardial infarction: a case report. Blood Coagul Fibrinolysis. 1999; 10:439–442.
5. Elkayam O, Paran D, Milo R, et al. Acute myocardial infarction associated with high dose intravenous immunoglobulin infusion for autoimmune disorders. A study of four cases. Ann Rheum Dis. 2000; 59:77–80.
6. Khandekar MM, Khurana AS, Deshmukh SD, Kakrani AL, Katdare AD, Inamdar AK. Platelet volume indices in patients with coronary artery disease and acute myocardial infarction: an Indian scenario. J Clin Pathol. 2006; 59:146–149.
7. Patrassi GM, Sartori MT, Rigotti P, et al. Coagulation and fibrinolysis during the first year of immunosuppressive treatment in renal transplantation: correspondence between hypercoagulable state and steroid therapy. Clin Appl Thromb Hemost. 1995; 1:277–282.
8. Stangel M, Hartung HP, Marx P, Gold R. Side effects of high-dose intravenous immunoglobulins. Clin Neuropharmacol. 1997; 20:385–393.
9. Fisman DN, Smilovitch M. Intravenous immunoglobulin, blood viscosity and myocardial infarction. Can J Cardiol. 1997; 13:775–777.
10. Caputo RP, Abraham S, Churchill D. Transradial coronary stent placement in a patient with severe idiopathic autoimmune thrombocytopenic purpura. J Invasive Cardiol. 2000; 12:365–368.
11. Moretti C, Teresa Lucciola M, Morena L, et al. Idiopathic thrombocytopenic purpura and percutaneous coronary stenting: a dangerous duo? Int J Cardiol. 2008; 130:e96–e97.
12. Fong MC, Chen KC, Leu HB, Chen LC. Coronary revascularization in a patient with immune thrombocytopenic purpura. J Chin Med Assoc. 2006; 69:436–438.
13. Neskovic AN, Stankovic I, Milicevic P, et al. Primary PCI for acute myocardial infarction in a patient with idiopathic thrombocytopenic purpura. A case report and review of the literature. Herz. 2010; 35:43–49.
14. Agostoni P, Biondi-Zoccai GG, de Benedictis ML, et al. Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures; Systematic overview and meta-analysis of randomized trials. J Am Coll Cardiol. 2004; 44:349–356.
15. Russo A, Cannizzo M, Ghetti G, et al. Idiopathic thrombocytopenic purpura and coronary artery disease: comparison between coronary artery bypass grafting and percutaneous coronary intervention. Interact Cardiovasc Thorac Surg. 2011; 13:153–157.
16. Chevalier B, Lancelin B, Koning R, et al. Effect of a closure device on complication rates in high-local-risk patients: results of a randomized multicenter trial. Catheter Cardiovasc Interv. 2003; 58:285–291.
17. Yellin A, Refaely Y, Paley M, Simansky D. Major bleeding complicating deep sternal infection after cardiac surgery. J Thorac Cardiovasc Surg. 2003; 125:554–558.
18. Mathew TC, Vasudevan R, Leb L, Pezzella SM, Pezzella AT. Coronary artery bypass grafting in immune thrombocytopenic purpura. Ann Thorac Surg. 1997; 64:1059–1062.
19. Inoue Y, Lim RC, Nand P. Coronary artery bypass grafting in an immune thrombocytopenic purpura patient using off-pump techniques. Ann Thorac Surg. 2004; 77:1819–1821.
20. Ascione R, Williams S, Lloyd CT, Sundaramoorthi T, Pitsis AA, Angelini GD. Reduced postoperative blood loss and transfusion requirement after beating-heart coronary operations: a prospective randomized study. J Thorac Cardiovasc Surg. 2001; 121:689–696.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download