Abstract
Background and Objectives
The inhibition of cholesterol absorption by ezetimibe increases cholesterol synthesis. The effect of inhibition of cholesterol synthesis on cholesterol absorption is controversial. The influence of these interactions on cholesterol levels is unknown. We investigated on the extent to which cholesterol levels were affected by the reaction of one pathway to the inhibition of the other pathway.
Subjects and Methods
This case-controlled study enrolled 198 patients who needed cholesterol-lowering drugs. Ezetimibe (10 mg) was administered to the patients with (n=58) and without on-going statin therapy (n=58). Simvastatin (20 mg) was administered to the patients treated with (n=41) and without ezetimibe (n=41).
Results
Ezetimibe without statin lowered the total cholesterol by 13.3±8.8% (p<0.001) and the low density lipoprotein-cholesterol (LDL-C) by 18.7±15.3% (p<0.001). Ezetimibe added to statin decreased the total cholesterol by 21.1±7.7% (p<0.001) and the LDL-C by 29.9±12.6% (p<0.001). The total cholesterol and LDL-C were reduced more by ezetimibe in patients with statin therapy than in those without statin therapy (p<0.001 and p<0.001, respectively). The differences in the effect of simvastatin on total cholesterol and LDL-C between the patients with and without ezetimibe showed borderline significance (p=0.10 and p=0.055, respectively).
Ezetimibe, an inhibitor of intestinal cholesterol absorption, lowers the low density lipoprotein-cholesterol (LDL-C) by 17-18%.1)2)3)4) However, ezetimibe elevates the activity of 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase resulting in the increase of cholesterol synthesis.5)6)7) When ezetimibe was added to an on-going statin therapy, LDL-C was reduced by 25-27%.8)9)10) The difference in the reduction of LDL-C suggests that the effect of ezetimibe on cholesterol levels may be weakened due to increased cholesterol synthesis. However, there are no reports that have directly evaluated the extent to which the increased cholesterol synthesis affects cholesterol levels. The effect of inhibition of HMG CoA reductase on cholesterol absorption is controversial.6)7)11)12)13) It is unknown whether the effect of statin on cholesterol absorption influences cholesterol levels. The aim of this study was to directly assess the effect of interactions between statin and ezetimibe on blood cholesterol levels.
The study protocol was approved by the Institutional Review Board of Chung-Ang University Hospital {IRB No. C2011168(618)}. Informed consent was waived by the IRB.
This case-controlled study enrolled 198 patients who did not achieve the goal of the Adult Treatment Panel III of the National Cholesterol Education Program. The exclusion criteria were as follows: 1) new onset of diseases that can influence lipid levels, such as diabetes mellitus, infectious diseases, or other endocrinologic diseases, within the last 3 months; 2) aspartate aminotransferase or alanine aminotransferase levels ≥3-fold of the upper normal limit; and 3) starting of new medications within the last 3 months that can affect lipid levels.
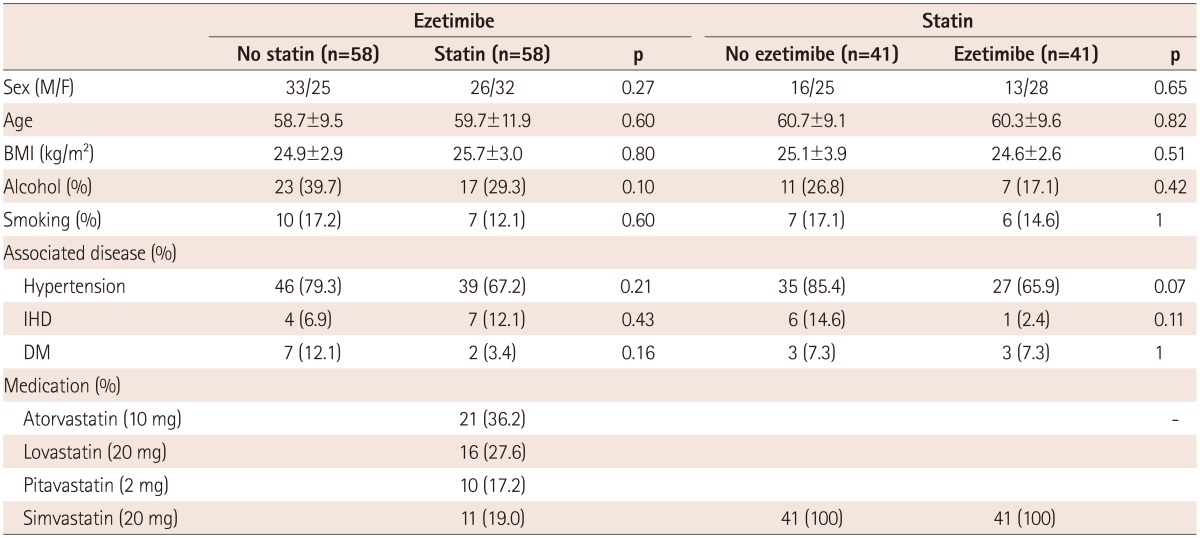
Ezetimibe (10 mg) was administered to patients without on-going statin therapy (n=58) and with on-going statin therapy (n=58). The on-going statins were atorvastatin (10 mg, n=21), lovastatin (20 mg, n=16), pitavastatin (2 mg, n=10), and simvastatin (20 mg, n=11) (Table 1). Simvastatin (20 mg) was administered to patients without on-going ezetimibe therapy (n=41) and with on-going ezetimibe therapy (10 mg, n=41).
Lipid profiles were measured before and after the management for 2 months. The blood samples were obtained after overnight fasting. The concentrations of total cholesterol and triglyceride were determined by the enzymatic method using an automatic analyzer (Model 7150, Hitachi, Tokyo, Japan). LDL-C was calculated with the Friedewald equation. The concentration of high density lipoprotein-cholesterol (HDL-C) was measured by direct method using immunoinhibition (Wako Pure Chemical Industries, Ltd., Osaka, Japan).
The data are expressed as mean±SD. The statistical analysis was performed using the Social Package for the Social Sciences (version 9.0, SPSS, Inc., Chicago, IL, USA). The paired t-test was used to compare concentrations before and after the medications, and the Student t-test was used to evaluate the differences between the groups. For triglyceride, the Wilcoxon signed-rank test was used to compare concentrations before and after the therapy, and the Mann-Whitney U test was used to evaluate the differences between the groups. The distribution of discrete variables was analyzed using the χ2 test. Differences among the groups were analyzed by the Kruskal-Wallis method. A two-tailed p<0.05 was considered statistically significant.
The baseline demographic and clinical characteristics were similar between the patients with and without on-going statin therapy of the ezetimibe-administered group, and between the patients with and without on-going ezetimibe of the simvastatin-administered group (Table 1). The baseline values of total cholesterol, LDL-C, HDL-C, and triglyceride levels were also similar between the groups (Table 2).
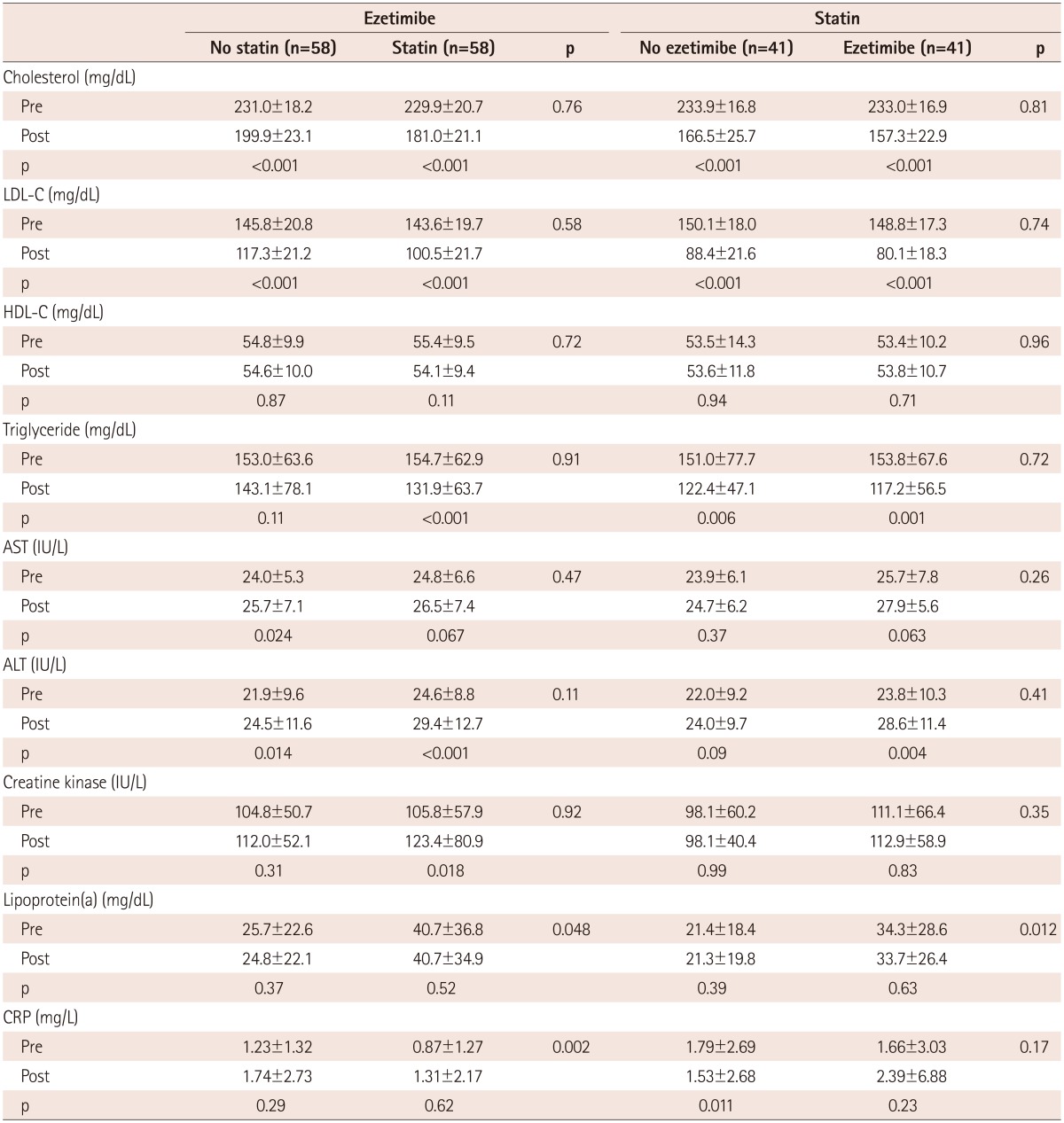
Ezetimibe lowered total cholesterol by 13.3±8.8% (from 231.0±18.2 to 199.9±23.1 mg/dL, p<0.001) in patients without on-going statin therapy and by 21.1±7.7% (from 229.9±20.7 to 181.0±21.1 mg/dL, p<0.001) in patients with on-going statin therapy (Table 2). This difference was statistically significant (p<0.001) (Fig. 1).
Ezetimibe lowered LDL-C by 18.7±15.3% (from 145.8±20.8 to 117.3±21.2 mg/dL, p<0.001) in patients without on-going statin therapy and by 29.9±12.6% (from 143.6±19.7 to 100.5±21.7 mg/dL, p<0.001) in patients with on-going statin therapy (Table 2). The reduction in LDL-C was greater in patients with on-going statin therapy than in those without on-going statin therapy (p<0.001) (Fig. 1).
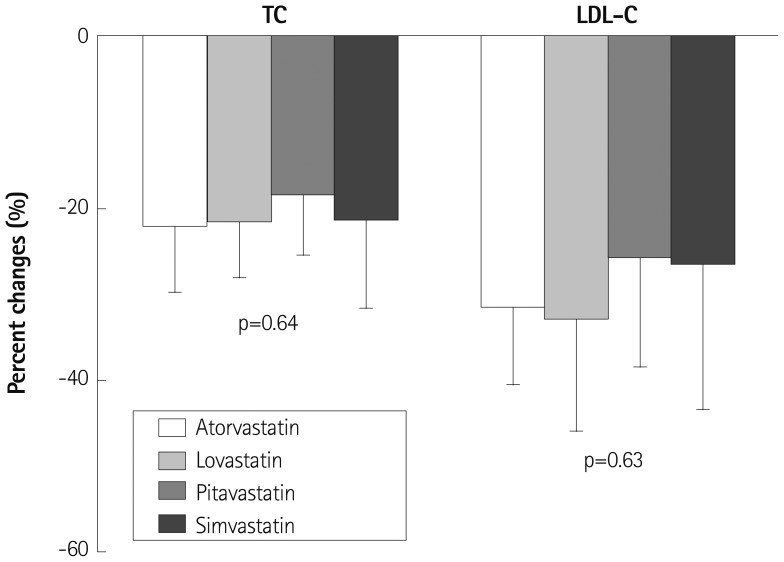
In the patients with on-going statin therapy, the effect of ezetimibe on the total cholesterol (p=0.64) and the LDL-C (p=0.63) levels was not significantly different among the on-going medication subgroups of atorvastatin, lovastatin, pitavastatin, and simvastatin (Fig. 2). The patients with on-going lovastatin therapy (n=16) showed similar changes in total cholesterol (p=0.84) and LDL-C (p=0.89) levels compared to those with on-going atorvastatin, pitavastatin, and simvastatin therapy (n=42).
Simvastatin reduced total cholesterol by 28.9±8.7% (from 233.9±16.8 to 166.5±25.7 mg/dL, p<0.001) in the patient groups that are not taking ezetimibe and by 32.6±7.8% (from 233.0±16.9 to 157.3±22.9 mg/dL, p<0.001) in the patient groups that are taking ezetimibe (Table 2).
Simvastatin decreased LDL-C by 41.1±12.3% (from 150.1±18.0 to 88.4±21.6 mg/dL, p<0.001) in patient groups that are not taking ezetimibe and by 46.1±11.3% (from 148.8±17.3 to 80.1±18.3 mg/dL, p<0.001) in the patient groups that are taking ezetimibe. The differences in the reductions of total cholesterol and LDL-C were of borderline significance (p=0.10 and p=0.055, respectively) (Fig. 1).
There were no changes in the HDL-C and lipoprotein levels (a) in all groups. The triglyceride level decreased in all groups, except for the only ezetimibe-administered group (p=0.11) (Table 2). The C-reactive protein level decreased in the only statin-administered group (p=0.011).
This case-controlled study demonstrated that the inhibition of cholesterol absorption by ezetimibe lowered the total cholesterol and LDL-C levels more in patients with on-going statin therapy than in those without on-going statin therapy. This finding suggests that the increase in cholesterol synthesis, due to the inhibition of cholesterol absorption, results in a significant elevation of the cholesterol and LDL-C levels. To the best of our knowledge, this is the first report that directly evaluated the extent to which the increased cholesterol synthesis affects cholesterol levels.
Several studies have reported that ezetimibe increases cholesterol synthesis, which was measured using isotopes or the surrogate markers of cholesterol synthesis, such as the ratio of lathosterol, desmosterol, or cholestenol to cholesterol.5)6)7) However, no studies have investigated the direct effect of this interaction on cholesterol levels. In the present study, a prior inhibition of cholesterol synthesis by statin enhanced the effect of lowering the total cholesterol and LDL-C levels with ezetimibe by 7.8% (p<0.001) and 11.2% (p<0.001), respectively. This finding suggests that the increase in cholesterol synthesis induced by ezetimibe plays a significant role in weakening the cholesterol-lowering effect of ezetimibe, which in turn elevates the cholesterol levels. Comparing to 13-109% of increase in cholesterol synthesis with ezetimibe, the effect on cholesterol levels was rather small.5)6)7)
Statin cannot completely inhibit hydroxymethlyglutaryl-coenzyme A (HMG CoA) reductase. In addition, the effect of co-administration of ezetimibe and statin on cholesterol synthesis is controversial. Several investigations have reported that the co-administration does not alter cholesterol synthesis.6) However, other studies have suggested that cholesterol synthesis increases even in the co-administration of ezetimibe and statin.7) Therefore, the actual elevation of cholesterol levels with the increased cholesterol synthesis may be higher than what was demonstrated in this paper.
Ezetimibe lowered LDL-C by 17-18%.1)2)3)4) However, LDL-C was reduced by 25-27% when ezetimibe was added to an on-going statin therapy.8)9)10)14) In the present study, ezetimibe decreased LDL-C by 18.7±15.3% in the patients without on-going statin therapy and by 29.9±12.6% in the patients with on-going statin therapy. Although the cholesterol lowering effect of ezetimibe was greater in this study than in the previous studies, the difference of 11.2% in the effect of ezetimibe between the patients with and without on-going statin therapy is consistent with those of the previous studies. A greater cholesterol-lowering effect in this study may be partially explained by the racial differences. The previous studies were conducted in the Caucasian populations, and this study was done in Asian population with relatively lower body surface area.
The co-administration of ezetimibe and statin produces an incremental reduction of LDL-C in the range of 12% to 15% compared to single statin therapy.1)2)3)4) As shown above, LDL-C was reduced by 25-27% when ezetimibe was added to an on-going statin therapy.8)9)10)14) This difference may be caused by the method used for calculating the change in cholesterol levels. For example, if statin decreases LDL-C from 200 mg/dL to 120 mg/dL and the addition of ezetimibe incrementally reduced to 94 mg/dL, the reduction of 26 mg/dL by ezetimibe is 13% of the initial LDL-C level (=26/200×100). However, this reduction is 21.7% (=26/120×100) of LDL-C level after statin therapy. Therefore, the calculation method masks the effect of ezetimibe on cholesterol levels, due to increased cholesterol synthesis, and the effect of combined therapy of statin and ezetimibe on cholesterol levels is not additive but synergistic.
Statin reduces cardiovascular events not only by reducing cholesterol biosynthesis but also by the so-called pleiotropic effects.15) The addition of ezetimibe failed to improve the pleiotropic effect of statin, in spite of a markedly further reduction of LDL-C.16)17)18)19) Therefore, ezetimibe may weaken the benefits of statin beyond the cholesterol reduction, by increasing HMG-CoA reductase activity which results in the elevation of cholesterol levels.
The effects of combined therapy of statin and ezetimibe on the surrogate markers of cardiovascular diseases were disappointing.14)20)21)22)23) The combined therapy reduced LDL-C comparable to or even more than the effects of higher dose of statin therapy or statin plus niacin. However, the primary endpoints were not different or were even worse in the combined therapy. These results might partially be related to the increase in HMG-CoA reductase activity by ezetimibe, which is confirmed through its effect on cholesterol levels in the present study.
The degree of inhibition of HMG-CoA reductase is variable according to the type and dose of statins. Usually, 20 mg of lovastatin is less effective in lowering cholesterol levels compared with other statins used in the present study. Therefore, it may be possible that ezetimibe reduces cholesterol levels to a lesser extent in the patients with on-going lovastatin therapy than in those with other on-going statin therapies. However, there were no significant differences in changes in total cholesterol and LDL-C among the subgroups of the on-going statin medications (p=0.64 and p=0.63, respectively) and between patients with on-going lovastatin therapy and those with other on-going statin therapies (p=0.84 and p=0.39, respectively). This finding suggests that the increase in cholesterol synthesis by ezetimibe may be completely blocked independent of the degree of inhibition of HMG-CoA reductase. Because each subgroup had a relatively smaller number of patients, further studies are needed to confirm these results.
The effect of statin on cholesterol absorption is controversial.6)7)11)12)13) The previous papers have reported that statin increases, does not alter, or decreases the cholesterol absorption. However, there were no reports that observed the effect of this interaction on cholesterol levels. In the present study, the pre-treatment with ezetimibe showed an increasing trend in the cholesterol and LDL-C lowering effect of statin by 3.7% (p=0.10) and 5.0% (p=0.055), respectively, although these changes did not reach statistical significance. This finding suggests that statin may induce a small increase in cholesterol absorption.
There are several limitations in this study. This study was performed at a single hospital. As this study made baseline lipid profiles well-matched between patients with and without lipid-lowering medications, there was a possibility of inhomogeneity of patients between the groups.
References
1. Dujovne CA, Ettinger MP, McNeer JF, et al. Efficacy and safety of a potent new selective cholesterol absorption inhibitor, ezetimibe, in patients with primary hypercholesterolemia. Am J Cardiol. 2002; 90:1092–1097. PMID: 12423709.
2. Knopp RH, Gitter H, Truitt T, et al. Effects of ezetimibe, a new cholesterol absorption inhibitor, on plasma lipids in patients with primary hypercholesterolemia. Eur Heart J. 2003; 24:729–741. PMID: 12713767.
3. Ballantyne CM, Houri J, Notarbartolo A, et al. Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation. 2003; 107:2409–2415. PMID: 12719279.
4. Sager PT, Melani L, Lipka L, et al. Effect of coadministration of ezetimibe and simvastatin on high-sensitivity C-reactive protein. Am J Cardiol. 2003; 92:1414–1418. PMID: 14675576.
5. Sudhop T, Lütjohann D, Kodal A, et al. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation. 2002; 106:1943–1948. PMID: 12370217.
6. Gouni-Berthold I, Berthold HK, Gylling H, et al. Effects of ezetimibe and/or simvastatin on LDL receptor protein expression and on LDL receptor and HMG-CoA reductase gene expression: a randomized trial in healthy men. Atherosclerosis. 2008; 198:198–207. PMID: 17980884.
7. Sudhop T, Reber M, Tribble D, et al. Changes in cholesterol absorption and cholesterol synthesis caused by ezetimibe and/or simvastatin in men. J Lipid Res. 2009; 50:2117–2123. PMID: 19380898.
8. Gagné C, Bays HE, Weiss SR, et al. Efficacy and safety of ezetimibe added to ongoing statin therapy for treatment of patients with primary hypercholesterolemia. Am J Cardiol. 2002; 90:1084–1091. PMID: 12423708.
9. Pearson TA, Denke MA, McBride PE, Battisti WP, Brady WE, Palmisano J. A community-based, randomized trial of ezetimibe added to statin therapy to attain NCEP ATP III goals for LDL cholesterol in hypercholesterolemic patients: the ezetimibe add-on to statin for effectiveness (EASE) trial. Mayo Clin Proc. 2005; 80:587–595. PMID: 15887425.
10. Pearson TA, Ballantyne CM, Veltri E, et al. Pooled analyses of effects on C-reactive protein and low density lipoprotein cholesterol in placebo-controlled trials of ezetimibe monotherapy or ezetimibe added to baseline statin therapy. Am J Cardiol. 2009; 103:369–374. PMID: 19166691.
11. Uusitupa MI, Miettinen TA, Happonen P, et al. Lathosterol and other noncholesterol sterols during treatment of hypercholesterolemia with lovastatin alone and with cholestyramine or guar gum. Arterioscler Thromb. 1992; 12:807–813. PMID: 1319735.
12. Vanhanen H, Miettinen TA. Pravastatin and lovastatin similarly reduce serum cholesterol and its precursor levels in familial hypercholesterolaemia. Eur J Clin Pharmacol. 1992; 42:127–130. PMID: 1618241.
13. Hidaka H, Kojima H, Kawabata T, et al. Effects of an HMG-CoA reductase inhibitor, pravastatin, and bile sequestering resin, cholestyramine, on plasma plant sterol levels in hypercholesterolemic subjects. J Atheroscler Thromb. 1995; 2:60–65. PMID: 9225210.
14. Taylor AJ, Villines TC, Stanek EJ, et al. Extended-release niacin or ezetimibe and carotid intima-media thickness. N Engl J Med. 2009; 361:2113–2122. PMID: 19915217.
15. Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004; 109(23 Suppl 1):III39–III43. PMID: 15198965.
16. Liu PY, Liu YW, Lin LJ, Chen JH, Liao JK. Evidence for statin pleiotropy in humans: differential effects of statins and ezetimibe on rho-associated coiled-coil containing protein kinase activity, endothelial function, and inflammation. Circulation. 2009; 119:131–138. PMID: 19075102.
17. Piorkowski M, Fischer S, Stellbaum C, et al. Treatment with ezetimibe plus low-dose atorvastatin compared with higher-dose atorvastatin alone: is sufficient cholesterol-lowering enough to inhibit platelets? J Am Coll Cardiol. 2007; 49:1035–1042. PMID: 17349882.
18. Fichtlscherer S, Schmidt-Lucke C, Bojunga S, et al. Differential effects of short-term lipid lowering with ezetimibe and statins on endothelial function in patients with CAD: clinical evidence for 'pleiotropic' functions of statin therapy. Eur Heart J. 2006; 27:1182–1190. PMID: 16621868.
19. Meaney A, Ceballos G, Asbun J, et al. The VYtorin on Carotid intima-media thickness and overall arterial rigidity (VYCTOR) study. J Clin Pharmacol. 2009; 49:838–847. PMID: 19443679.
20. Fleg JL, Mete M, Howard BV, et al. Effect of statins alone versus statins plus ezetimibe on carotid atherosclerosis in type 2 diabetes: the SANDS (Stop Atherosclerosis in Native Diabetics Study) trial. J Am Coll Cardiol. 2008; 52:2198–2205. PMID: 19095139.
21. Kastelein JJ, Akdim F, Stroes ES, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008; 358:1431–1443. PMID: 18376000.
22. Rossebø AB, Pedersen TR, Boman K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008; 359:1343–1356. PMID: 18765433.
Fig. 1
Comparison of percent changes in total cholesterol (TC) and low density lipoprotein-cholesterol (LDL-C) levels between the patients with and without on-going statin therapy of the ezetimibe-administered group (left), and between the patients with and without on-going ezetimibe therapy of the statin-administered group (right).
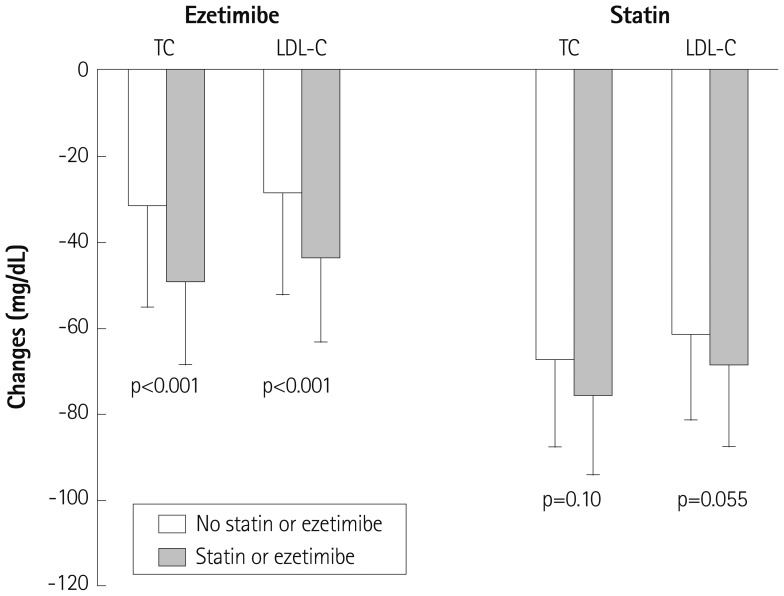
Fig. 2
Comparison of percent changes in cholesterol and low density lipoprotein-cholesterol (LDL-C) levels according to the on-going statin therapies in the ezetimibe-administered group. TC: total cholesterol.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download