Introduction
Coronary artery fistula (CAF) is defined as an abnormal direct connection between a coronary artery and one of the cardiac chambers or vascular structures. It is a rare anomaly with a reported incidence of less than 0.5% and a diagnosis rate of approximately 2% of all patients undergoing coronary angiography.1)2) It may not be a common condition, but CAF is clinically important in adulthood because of the increased risk of complications, such as heart failure, development of coronary artery diseases, aneurysmal changes, or ruptures of the communicating vessels.3) Here, we present a rare case of multidirectional CAFs accompanied by persistent left superior vena cava (PLSVC) and right-sided aortic arch in a patient who had surgical correction of tetralogy of Fallot (TOF) as a young adult.
Case
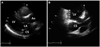
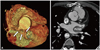
A 46-year-old male who had undergone cardiac surgery for TOF 24 years previous visited our hospital with symptoms of atypical chest pain and dyspnea of New York Heart Association class I. The patient was a current smoker but had no history of other underlying diseases. Normal sinus rhythm with first degree atrioventricular block was seen on the patient's electrocardiography, and his transthoracic echocardiography showed a dilated coronary sinus with the suggestion of possible PLSVC (Fig. 1). Mild mitral regurgitation and tricuspid regurgitation were seen, but only trivial pulmonary regurgitation was present. Pulmonary artery systolic pressure was within the normal range. A dilated right ventricle and atrium were identified without ventricular hypertrophy or functional abnormalities. Cardiac computed tomography revealed a fistula originating from the left main coronary artery and communicating with the left atrium (Fig. 2A). PLSVC with an aneurysmal change of the coronary sinus and right-sided aortic arch (Fig. 2B) were also seen with an enlarged right atrium and ventricle, compatible with the echocardiographic findings. However, the cause of dilation of the right side of the heart was not certain because there were no previous echocardiographic images to review; it may have been due to the patient's previous history of TOF or a result of PLSVC.
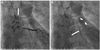
Coronary angiography was performed for the evaluation of the exact origin and communication of the fistula. Starting from the proximal part of the left main coronary artery, the fistula was directly communicating with the left atrium (Fig. 3A) and had two other branches. The upper lateral branch was connected with the pulmonary artery, and the lower descending branch was communicating with the subphrenic artery (Fig. 3B). Coronary blood flow was well preserved without significant stenosis in any coronary artery. Because the patient had only mild symptoms without any daytime functional disturbance, we decided to put the patient under close observation without specific intervention or medication.
Discussion
Coronary artery fistula was first described by Krause in 1865. With the development and improvement of coronary angiography, increasing numbers of coronary fistulas of different origins have been diagnosed and treated.4) The cause of this rare abnormality may be explained by the persistence of intratrabecular gaps. It is usually a congenital defect, but acquired fistulas also have been reported following various cardiac surgeries.3)5)
Coronary artery fistulas that arise from the right coronary artery and communicate with the right side of the heart are the types that have been most commonly reported in previous studies. Fistulas that arise from the left coronary arteries are known to be more rare than those that arise from the right, especially when they drain to the left side of the heart.1) Fistulas that originate from the left anterior descending artery are more commonly seen than those with an origin at the left circumflex artery; the prevalence of the latter remains uncertain due to its very uncommon nature.
The clinical symptoms and signs related to CAFs are variable. Most patients are asymptomatic, but in some cases, patients had symptoms such as fatigue, dyspnea, angina, and palpitation.6) The clinical presentation of CAFs that originate from the left coronary artery and drain to the left side of the heart mainly depends on the severity of the left-to-right shunt.7) Pressure overload of the left cardiac chambers may occur if the shunt through the fistula is large enough to be responsible for the variable clinical symptoms described previously.8) Coronary artery steal phenomenon, which is defined as a shunt flowing from the coronary artery to the relatively less-resistant fistula, causing decreased perfusion of the myocardial tissue distal to the coronary artery is another explanation for the development of angina or chest discomfort that may lead to complications, such as heart failure and myocardial ischemia.9)
In situations where CAFs present with large shunts or multiple complications, treatment is considered, but there are no definite interventional guidelines.10)11) Current recommendations are based on only some of the small retrospective studies or clinical case reports. The closure of the fistula with large shunts is recommended in symptomatic patients in order to prevent the development of complications.7) Surgery and direct ligation were the traditional method of treatment. However, with the development of catheter-based closure methods introduced in the early 1980s, trans-catheter closure of CAFs is currently carried out with good success.12) Variable techniques using balloons, coils, stents, and chemicals have also been developed.13)14)
In our report, the patient had multiple fistulas originating from the left main coronary artery and draining to the left atrium, with two branches communicating with the pulmonary artery and the subphrenic artery, respectively. There were no documents that indicated whether the patient underwent a coronary evaluation before the previous cardiac surgery for TOF, so it was uncertain whether these anomalies were congenital. In some cases, TOF was associated with CAFs, but most of them were small, only communicating with the pulmonary artery.15) Our patient also had PLSVC, which is a rare disease entity. The probability of one person having multiple congenital anomalies cannot be high; we could not find any previous reports of these kinds of multiple anomalies.
Although the patient had symptoms of atypical chest pain and dyspnea, these symptoms were not aggravated by exercise, so tests for a differential diagnosis, such as a treadmill test or 24-hour Holter monitoring, were not conducted. It was unlikely that the symptoms were related to the fistula because the size of the fistula was small, unable to form a large shunt, and presented no evidence of pressure overload on the left side of the heart upon echocardiography. Left ventricular hypertrophy was absent, and the E/E' was within normal range. The patient's symptoms spontaneously improved without medication, so further treatment was designed to be conservative.
It is important to identify CAFs in patients with TOF because the surgical strategy may differ according to its presence. Large CAFs must be ligated before commencing cardiopulmonary bypass to prevent loss of volume to the pulmonary vascular bed. Failure to close such communications may cause loss of the cardioplegia solution to the pulmonary circulation and incomplete myocardial protection during administration of cardioplegia after the aortic cross clamp is applied.16) Such possibilities emphasize the importance of coronary artery evaluation before cardiac surgery for TOF. In addition, only a few long-term observational studies regarding the prediction of the development of symptoms or complications in patients with CAFs currently exist. Further case reports or studies will be needed for risk and prognostic assessments of patients with such multiple coronary anomalies.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download