Abstract
Ante mortem cases of venous thrombosis in patients with nonbacterial thrombotic endocarditis (NBTE) have not yet been reported. We describe a rare case of NBTE in a patient with mesenteric vein thrombosis. A healthy 37-year-old man with abdominal pain and fever underwent emergency small bowel resection due to bowel ischemia resulting from mesenteric vein thrombosis. Transthoracic echocardiography revealed multiple mobile masses attached to the anterior leaflet of the mitral valves and their chordae tendineae. On suspicion of infective endocarditis, the cardiac masses were excised through open-heart surgery. However, pathologic reviews were compatible with NBTE. The patient was stable after the cardiac surgery and was treated with warfarin. Laboratory and imaging findings regarding his hypercoagulable condition were all negative.
Nonbacterial thrombotic endocarditis (NBTE) is a disease characterized by the presence of small sterile vegetations on cardiac valve leafleats.1) The vegetations are composed of fibrin, platelet aggregates and red blood cells without evidence of inflammation or bacteria.2) NBTE is a rare disease entity usually identified in patients with advanced stages of malignancy, infection or other systemic illnesses associated with hypercoagulable states.1) The diagnosis of NBTE is difficult because there are no pathognomonic symptoms or signs, and it is usually associated with life-threatening underlying conditions.1)2) Herein, we report a patient who was diagnosed with NTBE with an unusual clinical presentation: mesenteric venous thrombosis.
A healthy 37-year-old man visited our emergency room with fever, abdominal pain and bloody diarrhea, which had all started five days ago. He was a current smoker and had a 34 pack-year of smoking history. The patient's fever and diarrhea subsided through the use of acetaminophen and an anti-diarrheal agent, however, the abdominal pain persisted. At the initial examination, he was afebrile and his vital signs were unremarkable with a blood pressure of 147/94 mm Hg, a pulse rate of 79 beats/min and a body temperature of 36.3℃. His abdomen was distended, and there was guarding with tenderness and rebound tenderness.
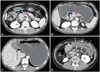
Inflammatory markers, including white blood cell count (19630/mm3) and C-reactive protein level (14.03 mg/dL), were markedly elevated. Abdominal computed tomography (CT) scan revealed small bowel infarction associated with acute mesenteric vein thrombosis. The proximal jejunum was edematous with ischemic segments. There were also portal vein thrombosis and a small amount of reactive ascites. No thrombi or emboli in the arterial system were identified on covered CT scan (Fig. 1). The patient underwent an emergent surgery for bowel resection. In the operative field, the small bowel mesenteric veins were occluded with thrombi, and the mesentery was edematous. A 120-cm length of small bowel with ischemic changes was resected. After the surgery, low molecular weight heparin was administered for anticoagulation.
Although the abdominal pain resolved after surgical resection of the ischemic bowel, the patient became febrile. His body temperature rose to 40℃. Laboratory examinations including blood culture, anti-nuclear antibody, anti-phospholipid antibody, anti-neutrophil cytoplasmic antibody, factor V Leiden mutation, homocysteine, factor XI, factor VIII, protein C, protein S, and anti-thrombin III were done, but they did not reveal any meaningful positive results. A disseminated intravascular coagulation (DIC) profile also did not show significant analytical findings {prothrombin time was 13.1 seconds (normal range: 10.7-11.9), activated partial thromboplastin time was 30.8 seconds (normal range: 19.6-36.0), fibrinogen was 366 mg/dL (normal range: 160-380), fibrinogen degradation product was 16.7 ug/mL (normal range: 0.0-4.9), and D-dimer was 5.61 mg/L (normal range: 0-0.55)}.
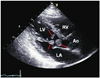
Electrocardiogram showed a normal sinus rhythm without ST-T segment or T wave changes. Transthoracic echocardiogram (TTE) was performed to investigate possible sources of cardiac disease. Surprisingly, three large hypermobile masses were found attached to the ventricular side of the anterior mitral valve leaflet and its chordae tendineae. Sizes of the masses were 16×16, 12×10, and 6×10 mm, respectively. TTE revealed normal left ventricular size and normal systolic function with 72% of ejection fraction. Mitral valvular function was normal without stenosis or regurgitation (Fig. 2). Malignancy evaluations with tumor markers and additional CT scans of the thorax were also unremarkable except for the masses in the left ventricle. On suspicion of infective endocarditis, serial blood cultures were performed again and empirical antibiotic therapy with ceftriaxone and gentamycin was started. However, the high fever persisted for the following 3 days.
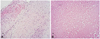
Due to the patient's poor response to antibiotics and high risk of systemic embolization, the cardiac masses were excised through an open-heart surgery. In the operative field, three masses were identified: one attached to the A2 portion of the ventricular side of the anterior mitral valve leaflet, another attached to the A2-A3 marginal chordae tendineae, and the last attached to the interventricular septum. These three masses were removed without valve repair or replacement surgery. Their gross appearances were indicative of vegetations (Fig. 3). The surgically resected masses were composed of necrotic fibrin thrombus and focal valvular tissues with fibromyxoid degeneration and minimal inflammatory reaction. Microscopically, there was no evidence of microorganism. These features were consistent with NBTE (Fig. 4). No microorganism was identified in the patient's serial blood culture studies as well. He was stable after the cardiac surgery and discharged with warfarin two weeks later. The patient has been on regular follow-up with no clinical evidence of disease for one year since the discharge.
Patients with NBTE tend to manifest with arterial thrombosis resulting in embolism and infarction in the peripheral organs. The spleen and kidney are known to be the most frequently affected organs. In addition, the brain was found to be commonly affected as well in previous studies where about half of the patients diagnosed with NBTE showed neurologic events caused by cerebral infarction.3)4) However, in this case, the small bowel was the only organ affected by thrombosis, and the small bowel infarction was caused not by arterial but by venous thrombosis. Although a previous autopsy study showed that about 7% of NBTE patients had mesenteric venous thrombosis,3) there has been no report of any ante mortem case of the condition.
While the pathogenesis of NBTE is not completely understood, previous studies have revealed that both endothelial damage and hypercoagulable states are important.1) Cardiac stress, trauma or circulating immune complexes can cause valvular damage. When deformed or sometimes normal valves are exposed to hypercoagulable states, such as malignant neoplasm, autoimmune diseases or DIC, thrombotic vegetations are formed.1)2)4) Therefore, patients diagnosed with NBTE are often associated with such conditions.1)2)3) In particular, malignancy is the most common underlying condition; 78% of NBTE patients were diagnosed with malignancy at autopsy,3) and there have been a few ante mortem case reports of NBTE associated with malignancy.5)6) Though a cause for the hypercoagulable milieu should have been identified in our NBTE patient, no evidence of an underlying condition was found despite our efforts.
In reaching the diagnostic conclusion of NBTE, we excluded infective endocarditis. First and foremost, it was not likely to be an infective endocarditis since there was no evidence of infection by a microorganism at all; all the blood culture results were negative. The patient did not respond to antibiotic therapies, and the pathologic specimen of the thrombi showed no evidence of infestation by microorganisms. Secondly, our patient's valves were structurally intact and functionally normal, while the valves in infective endocarditis are often destructed with or without abscess.7)
As part of the next step, left ventricular thrombus was excluded. In terms of location, left ventricular thrombus is usually seen in regions of blood stasis or low-velocity blood flow like ventricular aneurysms, which are not usually valves.8) Therefore, left ventricular thrombi are formed in an akinetic or a dyskinetic apex or in diffuse left ventricular dysfunction.8) The few reported cases of mural thrombi of the left ventricle with normal systolic function and without regional wall motion abnormality have been associated with hypercoagulable states, such as myeloproliferative disorder or eosinophilic endocarditis predisposing thrombus formation.9)10) In our case, one of the masses was attached to the left ventricular septum. Two other masses were attached to mitral valvular leaflet and its chordae tendineae. Our patient experienced no ischemic cardiac insult of any kind, and the heart function was too normal for an area of blood stasis to occur.
In our case, the sizes of the cardiac masses were 16×16, 12×10, and 6×10 mm, respectively, which were not quite typical of NBTE. Previous studies have reported that, the vegetations of NBTE were usually less than 10 mm1)2)3) and affected the atrial surfaces of the mitral valves and the ventricular surfaces of the aortic valves.1) However, none of these characteristics are independently diagnostic.8) For example, an autopsy study demonstrated vegetations of NBTE whose diameters ranged between 2 and 25 mm.11) In addition, some case reports showed vegetations of NBTE larger than 10 mm12)13) and a vegetation attached to a chordal structure.5) Therefore, our case cannot be ruled out of the boundaries of NBTE simply because of unusual sizes and locations of the vegetations.
The treatment of NBTE consists of systemic anticoagulation and control of the underlying disease. Since most of the underlying diseases of NBTE are malignancies, unfractionated heparin is recommended for anticoagulation.1)4) However, there is no guideline regarding the duration and methods of anticoagulation therapy for NBTE patients without cancer.4) Our patient chose warfarin over heparin for the sake of convenience. Given that our patient has been on follow-up without evidence of new thrombosis, a vitamin K antagonist might also be as effective as low molecular weight heparin. Indications of cardiac surgery in NBTE have not yet been established. Based upon our results, cardiac surgery should be considered in selected patients who have curable underlying diseases, severe valvular dysfunction or recurrent embolic events despite anticoagulation therapy.14)
We described the first case of NBTE without evidence of arterial thrombosis or underlying systemic illnesses. Until recently, ante mortem cases of NBTE have been scarcely reported. However, with the gradual progress and improvement of imaging techniques, more ante mortem cases of NBTE are expected to be identified. Cardiac masses that are not quite fit for being diagnosed as infected vegetations, left ventricular thrombi or tumors should be suspected of vegetations of NBTE. Prompt diagnosis and treatment of NBTE could improve the overall outcome of the treatment and prognosis of the disease by reducing complications.
Figures and Tables
Fig. 1
Computed tomographic scan of the abdomen demonstrating small bowel ischemia associated with mesenteric venous thrombosis. Thrombosis at the superior mesenteric and splenic veins (blue arrows) with an intact superior mesenteric artery (red arrow) (A and B), thrombosis at the portal vein (blue arrow) (C) and edematous proximal jejunum with ischemic changes (white arrows) (D).
Acknowledgments
The authors thank Jae-Sung Choi, Rumi Shin, and Jin Hyun Park for their helpful suggestions for and discussions regarding the manuscript.
References
1. Lopez JA, Ross RS, Fishbein MC, Siegel RJ. Nonbacterial thrombotic endocarditis: a review. Am Heart J. 1987; 113:773–784.
2. Asopa S, Patel A, Khan OA, Sharma R, Ohri SK. Non-bacterial thrombotic endocarditis. Eur J Cardiothorac Surg. 2007; 32:696–701.
3. Deppisch LM, Fayemi AO. Non-bacterial thrombotic endocarditis: clinicopathologic correlations. Am Heart J. 1976; 92:723–729.
4. el-Shami K, Griffiths E, Streiff M. Nonbacterial thrombotic endocarditis in cancer patients: pathogenesis, diagnosis, and treatment. Oncologist. 2007; 12:518–523.
5. Aryana A, Esterbrooks DJ, Morris PC. Nonbacterial thrombotic endocarditis with recurrent embolic events as manifestation of ovarian neoplasm. J Gen Intern Med. 2006; 21:C12–C15.
6. Norisada K, Tanaka H, Onishi T, et al. Nonbacterial thrombotic endocarditis associated with cancer of unknown origin complicated with thrombus in the left auricular appendage: case report. Cardiovasc Ultrasound. 2011; 9:8.
7. Habib G, Badano L, Tribouilloy C, et al. Recommendations for the practice of echocardiography in infective endocarditis. Eur J Echocardiogr. 2010; 11:202–219.
8. Otto CM. Textbook of Clinical Echocardiography. 5th ed. Philadelphia: Elsevier/Saunders;2013. p. 202–219.
9. Sivasankaran S, Harikrishnan S, Tharakan JM. Left ventricular thrombi in the presence of normal left ventricular function. Indian Heart J. 2002; 54:196–198.
10. Stoddard MF, Pearson AC, Kanter KR, Labovitz AJ. Left ventricular thrombus with normal left ventricular wall motion in a patient with myelofibrosis. Am Heart J. 1989; 117:966–968.
11. Llenas-García J, Guerra-Vales JM, Montes-Moreno S, et al. [Nonbacterial thrombotic endocarditis: clinicopathologic study of a necropsy series]. Rev Esp Cardiol. 2007; 60:493–500.
12. Lee V, Gilbert JD, Byard RW. Marantic endocarditis - A not so benign entity. J Forensic Leg Med. 2012; 19:312–315.
13. Vinales KL, Gopalan RS, Lanza LA, Lester SJ, Chaliki HP. Unusual case of nonbacterial thrombotic endocarditis attributable to primary antiphospholipid syndrome. Circulation. 2010; 122:e459–e460.
14. Rabinstein AA, Giovanelli C, Romano JG, Koch S, Forteza AM, Ricci M. Surgical treatment of nonbacterial thrombotic endocarditis presenting with stroke. J Neurol. 2005; 252:352–355.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download