Abstract
Background and Objectives
Vascular surgery carries high operative risk. Recently developed cardiac computed tomography (CT) provides excellent imaging of coronary artery disease (CAD), as well as myocardial perfusions. We investigated the role of stress perfusion CT with coronary computed tomography angiography (CCTA) using 128-slice dual source CT (DSCT) in preoperative cardiac risk evaluation.
Subjects and Methods
Patients scheduled for vascular surgery were admitted and underwent the adenosine stress perfusion CT with CCTA using DSCT. Patients who presented with unstable angina, recent myocardial infarction, decompensated heart failure, or renal failure were excluded. Stress perfusion CT was first acquired using sequential mode during adenosine infusion, after which, scanning for CT angiography was followed by helical mode. Perioperative events were followed up for 1 month.
Results
Ninety-one patients completed the study. Most patients (94.5%) had coronary atherosclerosis, with 36 (39.6%) patients had more than 50% coronary artery stenosis. Perfusion defects with significant stenosis were found in 12 cases (13.2%). Revascularization after DSCT was rarely performed. Four patients (4.4%) experienced cardiac events in the perioperative period: two experienced heart failure and two had non-fatal myocardial infarction.
Conclusion
We cannot conclude that the stress perfusion CT, with CCTA using DSCT, plays a significant role in preoperative risk evaluation from this study. However, the coronary atherosclerosis and the significant CAD were commonly found. The perfusion defects with significant lesions were found in only small fraction of the patients, and did not contribute to perioperative myocardial infarction or heart failure.
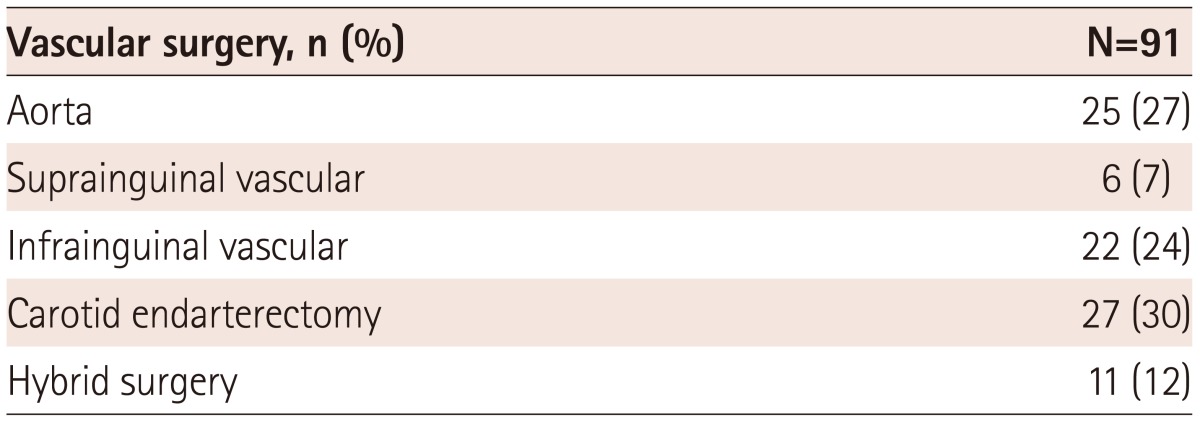
The preoperative cardiac risk assessment is based on the individual and surgical risks. The vascular surgery, including carotid endarterectomy and endovascular aneurysm repair, carries intermediate surgical risk, while the aortic/major vascular surgery and peripheral vascular surgery carry higher risk.1) The patients who undergo vascular surgery are more likely to develop perioperative cardiovascular events, as most vascular diseases share risk factors with coronary artery disease (CAD). Therefore, the preoperative evaluation for CAD is recommended in high-risk patients planning to undergo vascular surgery.
Coronary computed tomography angiography (CCTA) has been used extensively in the diagnosis of CAD. CCTA is the most powerful noninvasive anatomical imaging method for CAD,2) however, it has limited information on the functions of coronary arteries (perfusion). The adenosine-stress perfusion computed tomography (CT) has been recently introduced and combined with CCTA, leading to improvement in the accuracy of cardiac CT for diagnosing CAD.3) In this study, we investigated on the role of stress perfusion CT with CCTA using 128-slice dual source CT (DSCT).
The patients scheduled for the elective vascular surgery were included in this study (age >20 year old). Those with renal dysfunction (serum creatinine >2.0), unstable vital signs, and contraindications for iodine contrast (allergy, anaphylactic shock) or adenosine (asthma, atrioventricular block) were excluded. Written informed consent was provided to the patients before performing CCTA and stress perfusion CT. This protocol was reviewed and approved by the Institutional Review Board of Samsung Medical Center.
All of the patients were assessed for the preoperative risk factors by a cardiology consultant and were under surveillance for postoperative cardiac events, through serial electrocardiogram (ECG) and cardiac enzyme measurements {creatine phosphokinase-MB (CK-MB) and troponin I} at 6, 12, and 24 hours after surgery. The cardiac events were defined as myocardial infarction, unstable angina, decompensated heart failure, or uncontrolled arrhythmia, within 1 month after surgery. The perioperative and postoperative events, occurring after 1 month of follow-up, were assessed by the electronic medical record reviews and outpatient clinic interviews. The follow-up schedules at the outpatient clinic were totally dependent on the attending physicians (most of them are vascular surgeons) and there were no pre-defined, additional cardiac imagings or functional tests.
Cardiac CT was performed according to the following protocol. All of the patients underwent cardiac CT using a DSCT system (SOMATOM Definition Flash, Siemens Medical Solution, Forchheim, Germany). Initially, the single-heartbeat CT on calcium scoring was acquired with the following parameters: 2×64×0.6 mm detector collimation, resulting in 2×128×0.6 mm sections via the z-axis flying focal spot technique; 280 ms gantry rotation time; 120 kV tube potential; and 80 reference mAs per rotation tube current-time product with automatic tube current modulation. The adenosinestress perfusion CT scan was performed after infusing adenosine (0.14 mg/kg/min) into the antecubital vein for 3 minutes.4) While maintaining infusion, the iodine contrast was also injected at 2 minutes and 54 seconds, after the start of adenosine infusion with 40-50 mL of iomeprol 350 mg I/mL (Bracco, Milan, Italy), followed by 40-50 mL of saline injected at 4-5 mL/sec. The CT perfusion scan was performed in dynamic acquisition mode for 30 seconds while maintaining adenosine infusion. A stress perfusion CT was performed by shuttling the table between the two alternating table positions, with ECG-triggered mode for every other R-R interval. The anatomic coverage of this imaging technique was 73 mm with a 10% overlap between both acquisition ranges for a given detector width of 38 mm. The 100 kV tube voltage and the automatic tube current modulation technique with 350 reference mAs per rotation were used for dynamic stress scan.
Immediately after the stress perfusion CT was performed, the adenosine infusion was discontinued. After a rest period of 5-10 minutes, as the patient's heart rate returned to the baseline, 0.4 mg nitroglycerin was administered sublingually, followed by CCTA one minute later. The CCTA was performed with retrospective ECG-gated helical mode and ECG-modulated tube current (100% radiation dose during target R-R interval and 4% radiation dose during remaining R-R interval).5)
Cardiac CT was reviewed by two specialists (one with 3 years and another with 4 years of stress perfusion CT experience), both of whom were blinded to each subject's clinical data and identifiers. The readers independently analyzed the images. Discordant findings were reconciled during the consensus readings. The CT images were analyzed for technical factors, coronary artery calcium score (CACS), coronary artery stenosis, and adenosine-induced CT perfusion of myocardium. The CACS was calculated only for those without coronary stenting. The coronary artery stenosis was first evaluated visually. Then, the observers calculated the percentage of diameter stenosis on vessel cross-section images using commercial software (Terarecon Intuition, version 4.4.7; TeraRecon Inc., Foster City, CA, USA). A significant stenosis was defined quantitatively as >50% of luminal narrowing. In the patients with previous revascularization, insignificant stenosis in the current CTA has been considered "insignificant". The image quality was evaluated on a patient-by-patient basis and classified as adequate (allowing for the presence of image-degrading artifacts related to motion, calcification, and noise, while still amenable to evaluation with moderate confidence) or poor (presence of image-degrading artifacts resulting in low-confidence evaluation).6)
For the myocardial perfusion image, the stress perfusion CT images were reconstructed at 280 ms of the R-R interval, using a B23f kernel. The images were read in the cardiac short-axis view at a 4-mm slice thickness with averaged reconstruction. For detection of subtle perfusion defects, the observers used a user-defined narrow window width and a window level setting, as described previously.7) The stress perfusion CT images were visually evaluated according to the 16 myocardial segments, excluding the apex.8) We also excluded the myocardial segments that covered less than 50% of the thickness of the myocardium due to limited scan coverage (73 mm). Among the hypoattenuated segments, those lesions associated with significant stenosis were considered as perfusion defects. The hypoattenuated segments without significant corresponding stenosis were considered artifacts. The blood flow and volume of the myocardium were calculated from dynamic perfusion datasets, using a commercially available workstation with dedicated software for myocardial perfusion analysis (Leonardo; Siemens Medical Solutions, Erlangen, Germany) (Fig. 1).4)
The effective radiation dose was calculated by multiplying the dose-length product by the conversion coefficient κ (κ=0.017 mSv/mGy/cm) for the chest.9)
We attempted to scan ninety-five patients with CCTA and stress perfusion CT. Four patients failed to complete the stress perfusion CT due to low heart rate (n=2), hypotension (n=1), and poor cooperation (n=1).
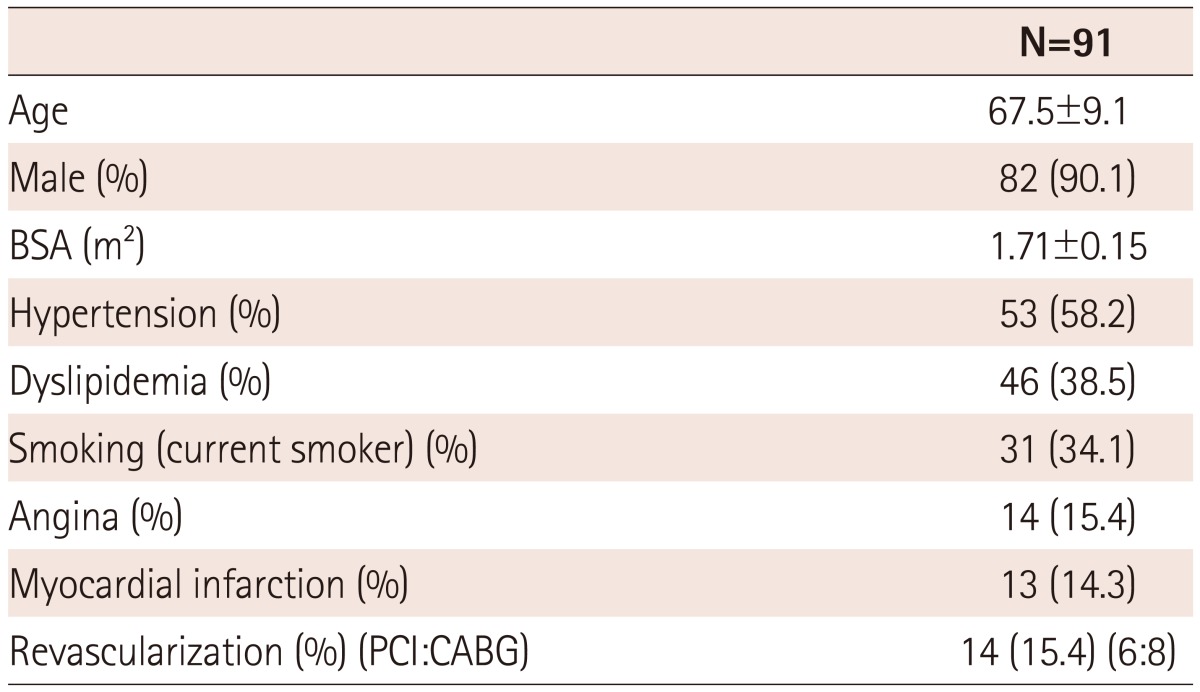
Thus, only ninety-one patients were analyzed. The characteristics of our study population are summarized in Table 1. The mean age was 68 years and males were the predominant sex (90.1%). More than half of the patients had hypertension (58%) and more than 30% had dyslipidemia and a smoking history. Previous revascularization or myocardial infarction were present in 15% of the patients.
The surgical procedures were categorized as aortic, suprainguinal and infrainguinal vascular surgery, carotid endarterectomy, and hybrid surgery (Table 2). Carotid endarterectomy (27%) and aortic surgery (25%) were the most common procedures in this study population.
The mean radiation dose was 791.5±427.25 mGy · cm and effective radiation dose for chest was 13.45±7.26 mSv. The average heart rate during the scan was 68.0±14.5 beats/minute. The total volume of iodine contrast used was 130±9 mL. CACS (Agaston score, density/sq.mm) was 186.0 (median) (473.5, interquartile range). The mean radiation dose for the stress perfusion CT and the coronary CTA were 427.4±261.2 mSv and 237.5±160.9 mSv. The image quality was adequate in 78 (85.7%) patients and poor in 13 (14.3%) patients.
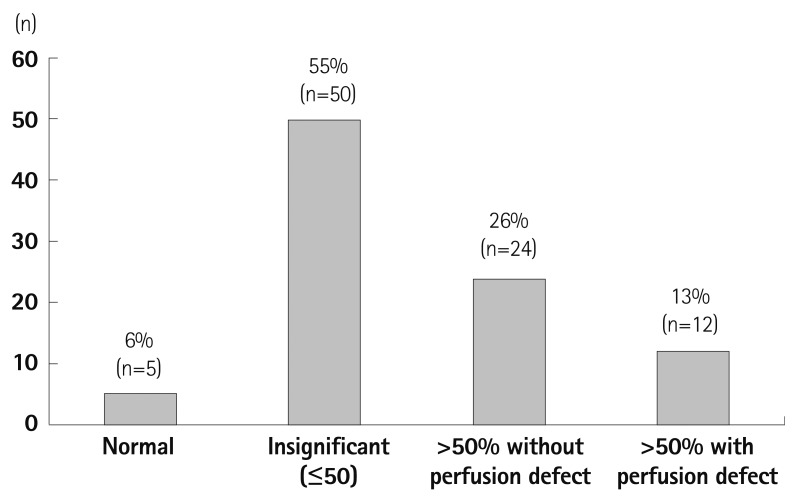
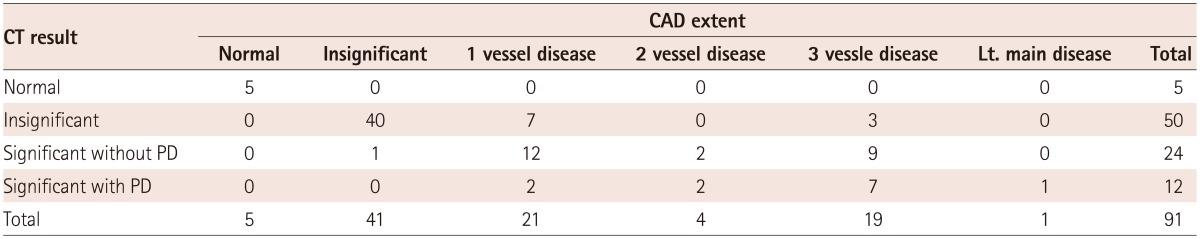
Extent of CAD, when analyzing CCTA only (Fig. 2), showed that 95% of the study population presented with coronary atherosclerosis. When significant CAD was defined as coronary artery stenosis and as more than 50% or already revascularized vessels, half of the patients (n=45, 49.4%) had CAD and about a third of the patients (n=28, 30.8%) had multivessel disease. To assess the significant CAD as a preoperative risk evaluation, the CT perfusion was analyzed with the CCTA. In this analysis (Fig. 3), the significant coronary stenosis was only defined as more than 50% in CCTA. The analysis showed that 26% (2/3 of coronary artery stenosis >50%) of the patients did not accompany perfusion defects and 13% (1/3 of coronary artery stenosis >50%) of the patients showed matched perfusion defects with coronary artery stenosis. (Fig. 3, Table 4).
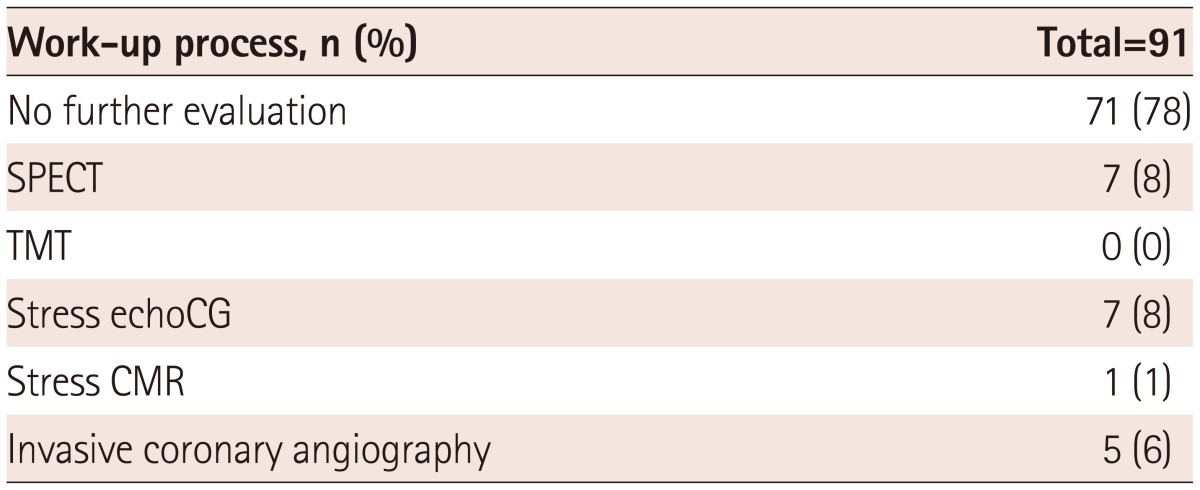
The pre-operative evaluation for CAD after cardiac CT was dependent on the attending cardiologists and the vascular surgeons. Twenty patients underwent additional work-up for CAD (Table 3). Only one patient underwent revascularization (bypass surgery) before vascular surgery, while others had surgery as scheduled, along with careful monitoring and beta-blocker treatment.
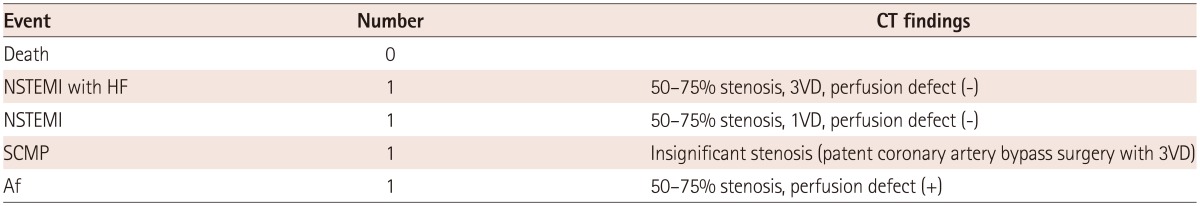
Overall, four patients (4.4%) experienced cardiovascular events (Table 5). There were no deaths perioperatively.
Two patients experienced perioperative, non-fatal MI. The first patient showed non-ST elevation myocardial infarction (NSTEMI) with heart failure symptoms. The preoperative CT findings showed three vessel diseases with significant coronary stenosis (50-75%), but no perfusion defects in the CT perfusion scan. This patient had low CACS (Agatston Score 7.15), and the CTA showed lipid-rich plaque with 60% stenosis in the left anterior descending artery and multifocal mild stenosis with remodeling by soft plaque in the right coronary artery.
The second patient had one vessel disease with significant coronary stenosis, also without perfusion defects in CT. Eccentric noncalcific plaque with more than 50% stenosis in the proximal to distal left anterior descending artery and segmental eccentric plaque with 50% stenosis in the proximal right coronary artery were found. This patient also had low CACS (Total Agatston Score 7.15). Two other patients developed post-operative heart failure, though the direct causes were defined as stress-induced cardiomyopathy and atrial fibrillation. The former had 3 disease vessels, but already had undergone a complete revascularization preoperatively, and was classified as an insignificant stenosis group. The latter had significant coronary artery stenosis (50-75%) with significant CT perfusion defects; this patient did not show elevated myocardial enzyme levels, and recovered after atrial fibrillation was controlled. Because these two patients had coexisting CAD, the possibility of triggering effect by myocardial ischemia to stress induced cardiomyopathy or atrial fibrillation cannot be ruled out.
In this study, the patients scheduled for vascular surgery underwent cardiac CT, including CCTA and stress perfusion CT. The cardiac CT showed that the coronary atherosclerosis and significant CAD was common in the patients scheduled for vascular surgery. Considering the high prevalence of CAD, the number of perioperative cardiovascular events was small, and the perfusion defects in stress perfusion CT did not predict perioperative myocardial infarction or heart failure.
Previous studies using the invasive coronary angiography have reported that 25% of the patients with vascular disease had correctable CAD.10) Baron et al.11) reported that 2.2% of the mortality had a cardiac etiology, with 19% experiencing the prolonged myocardial ischemia after abdominal aortic surgery. Nineteen percent of the subjects in this study had a history of angina, while 48% had hypertension. The mean age was 63 years, similar to that of our study population.
Considering the inclusion criteria only included the abdominal aortic surgeries, the incidence of postoperative cardiac events is much higher compared to our study where there were few cardiac events. This can be attributed to the following: first, there is a low incidence of cardiovascular events amongst Asians, as reported by a Japanese group;12) second, the type of hybrid surgery (endovascular repair) included in our study causes fewer cardiovascular events than does the open repair, although studies have shown that postoperative cardiac outcomes do not significantly differ between the two procedures.13)14)
For preoperative coronary risk evaluation, pharmacologic stress testing is usually preferred, because most patients struggle with physical exertion. Myocardial perfusion imaging15) or dobutamine stress echocardiography16) has been reported as useful methods for the preoperative coronary risk evaluation. The invasive coronary angiography and revascularization did not improve on short-term outcomes in the patients undergoing vascular surgery.17) Decision analysis without preoperative coronary angiography generally led to better outcomes, due to its high cost and complications involved with invasive coronary angiography. Furthermore, the coronary artery revascularization, before elective major vascular surgery, did not reduce the incidence of postoperative myocardial infarction and mid-term mortality.18) On the basis of previous studies, the best method for cardiac evaluation before the vascular surgery involves gathering both anatomical and functional data in a noninvasive manner.
The coronary CTA is a primary non-invasive technique to visualize the anatomical imaging for the coronary artery. To evaluate ischemic heart disease, the assessment of myocardial blood flow is helpful, such as single photon emission CT. The stress perfusion CT also can give information for the myocardial blood flow during rest and stress. Because of the higher radiation and contrast agent dosage, stress perfusion CT has been limited in the clinical practice. Recently, the development of new CT machine and protocols makes it much easier in clinical practice, with faster and lower radiation dose with high accuaracy.22)23)
The accuracy of perfusion CT has been evaluated with the several previous studies24)25); however, there are no previous stress perfusion CT studies for the preoperative cardiac risk evaluation in vascular surgery. Coronary atherosclerosis is common in the patients with severe vascular disease, so the coronary calcification is also frequently found in them. The high calcium score is associated with overestimation or as a source of artifact of coronary arterial stenosis in CTA, causing doubts in the usefulness of CTA in the patients preparing for vascular surgery. When accompanied by the stress perfusion CT as a functional imaging, the incremental value of cardiac CT has been reported where CTA and cardiac CT can still be applied to the vascular surgery patients, which improve the accuracy for cardiac risk assessment. In our study, about 40% of the patients had significant coronary artery stenosis and only a third of them had perfusion defect.
Interestingly, two patients, who experienced postoperative NSTEMI in our study, had low CACS, significant but moderate stenosis (less than 75%), and lipid-rich plaques with positive remodeling. However, they didn't have perfusion defects on stress CT. It might suggest that the plaque imaging in CCTA can be more helpful in predicting the postoperative cardiac events than the stress perfusion CT.
Although we did not prove the significant predictive value, because of the small number, our study suggests that the selection of high risk patients with true ischemia is possible even in high coronary calcium score.
The most important limitation in this study was the limited number of perioperarive cardiac events. This could be due to improvements in perioperative management, as well as a lower risk for such events in Asian ethnicities. The perfusion defect was not quantitatively evaluated in our study, since our aim was to evaluate its predictive value for the perioperative events. Future studies with higher statistical power might obtain quantitative measurements, in order to specify the risk groups as indicated by the magnitude of perfusion defects.
In conclusion, we cannot conclude from this study that the CCTA and stress perfusion CT using DSCT plays a significant role in the preoperative risk evaluation, due to the small sample size and low statistical power. However, coronary atherosclerosis and significant coronary artery stenosis were commonly found by CCTA in the patients scheduled for vascular surgery. The perfusion defects with significant lesions were found in only a small fraction of the patients, and they did not predict the perioperative myocardial infarction or the heart failure.
Acknowledgments
This study was supported by a grant from the Korean Society of Cardiology (2009-02).
References
1. Eagle KA, Berger PB, Calkins H, et al. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). J Am Coll Cardiol. 2002; 39:542–553. PMID: 11823097.
2. Hamon M, Biondi-Zoccai GG, Malagutti P, et al. Diagnostic performance of multislice spiral computed tomography of coronary arteries as compared with conventional invasive coronary angiography: a meta-analysis. J Am Coll Cardiol. 2006; 48:1896–1910. PMID: 17084268.
3. Feuchtner G, Goetti R, Plass A, et al. Adenosine stress high-pitch 128-slice dual-source myocardial computed tomography perfusion for imaging of reversible myocardial ischemia: comparison with magnetic resonance imaging. Circ Cardiovasc Imaging. 2011; 4:540–549. PMID: 21862731.
4. Kim SM, Kim YN, Choe YH. Adenosine-stress dynamic myocardial perfusion imaging using 128-slice dual-source CT: optimization of the CT protocol to reduce the radiation dose. Int J Cardiovasc Imaging. 2013; 29:875–884. PMID: 23076604.
5. Abbara S, Arbab-Zadeh A, Callister TQ, et al. SCCT guidelines for performance of coronary computed tomographic angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2009; 3:190–204. PMID: 19409872.
6. Chang SA, Choi SI, Choi EK, et al. Usefulness of 64-slice multidetector computed tomography as an initial diagnostic approach in patients with acute chest pain. Am Heart J. 2008; 156:375–383. PMID: 18657674.
7. Mahnken AH, Klotz E, Pietsch H, et al. Quantitative whole heart stress perfusion CT imaging as noninvasive assessment of hemodynamics in coronary artery stenosis: preliminary animal experience. Invest Radiol. 2010; 45:298–305. PMID: 20421799.
8. Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Int J Cardiovasc Imaging. 2002; 18:539–542. PMID: 12135124.
9. Einstein AJ, Moser KW, Thompson RC, Cerqueira MD, Henzlova MJ. Radiation dose to patients from cardiac diagnostic imaging. Circulation. 2007; 116:1290–1305. PMID: 17846343.
10. Hertzer NR, Beven EG, Young JR, et al. Coronary artery disease in peripheral vascular patients. A classification of 1000 coronary angiograms and results of surgical management. Ann Surg. 1984; 199:223–233. PMID: 6696538.
11. Baron JF, Mundler O, Bertrand M, et al. Dipyridamole-thallium scintigraphy and gated radionuclide angiography to assess cardiac risk before abdominal aortic surgery. N Engl J Med. 1994; 330:663–669. PMID: 8107716.
12. Seki M, Kashimoto S, Nagata O, et al. Are the incidences of cardiac events during noncardiac surgery in Japan the same as in the United States and Europe? Anesth Analg. 2005; 100:1236–1240. PMID: 15845660.
13. de Virgilio C, Bui H, Donayre C, et al. Endovascular vs open abdominal aortic aneurysm repair: a comparison of cardiac morbidity and mortality. Arch Surg. 1999; 134:947–950. discussion 950-1. PMID: 10487588.
14. De Bruin JL, Baas AF, Buth J, et al. Long-term outcome of open or endovascular repair of abdominal aortic aneurysm. N Engl J Med. 2010; 362:1881–1889. PMID: 20484396.
15. Boucher CA, Brewster DC, Darling RC, Okada RD, Strauss HW, Pohost GM. Determination of cardiac risk by dipyridamole-thallium imaging before peripheral vascular surgery. N Engl J Med. 1985; 312:389–394. PMID: 3871502.
16. Poldermans D, Fioretti PM, Forster T, et al. Dobutamine stress echocardiography for assessment of perioperative cardiac risk in patients undergoing major vascular surgery. Circulation. 1993; 87:1506–1512. PMID: 8491005.
17. Mason JJ, Owens DK, Harris RA, Cooke JP, Hlatky MA. The role of coronary angiography and coronary revascularization before noncardiac vascular surgery. JAMA. 1995; 273:1919–1925. PMID: 7783301.
18. McFalls EO, Ward HB, Moritz TE, et al. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med. 2004; 351:2795–2804. PMID: 15625331.
19. Russo V, Gostoli V, Lovato L, et al. Clinical value of multidetector CT coronary angiography as a preoperative screening test before noncoronary cardiac surgery. Heart. 2007; 93:1591–1598. PMID: 17164488.
20. Budde RP, Huo F, Cramer MJ, et al. Simultaneous aortic and coronary assessment in abdominal aortic aneurysm patients by thoraco-abdominal 64-detector-row CT angiography: estimate of the impact on preoperative management: a pilot study. Eur J Vasc Endovasc Surg. 2010; 40:196–201. PMID: 20427209.
21. Catalán P, Leta R, Hidalgo A, et al. Ruling out coronary artery disease with noninvasive coronary multidetector CT angiography before noncoronary cardiovascular surgery. Radiology. 2011; 258:426–434. PMID: 21079198.
22. Kim SM, Chang SA, Shin W, Choe YH. Dual-energy CT perfusion during pharmacologic stress for the assessment of myocardial perfusion defects using a second-generation dual-source CT: a comparison with cardiac magnetic resonance imaging. J Comput Assist Tomogr. 2014; 38:44–52. PMID: 24424556.
23. Kurata A, Kawaguchi N, Kido T, et al. Qualitative and quantitative assessment of adenosine triphosphate stress whole-heart dynamic myocardial perfusion imaging using 256-slice computed tomography. PLoS One. 2013; 8:e83950. PMID: 24376774.
24. Blankstein R, Shturman LD, Rogers IS, et al. Adenosine-induced stress myocardial perfusion imaging using dual-source cardiac computed tomography. J Am Coll Cardiol. 2009; 54:1072–1084. PMID: 19744616.
25. Rocha-Filho JA, Blankstein R, Shturman LD, et al. Incremental value of adenosine-induced stress myocardial perfusion imaging with dual-source CT at cardiac CT angiography. Radiology. 2010; 254:410–419. PMID: 20093513.
Fig. 1
Analysis of CTA and stress perfusion CT image. Patients with significant stenosis with stress induced perfusion defect in RCA territory. CTA shows tight stenosis in mid RCA (A and B), and the corresponding myocardial segments in basal inferior wall shows significant perfusion defect in stress perfusion CT image (C). CTA: computed tomographic angiography, CT: computed tomography, RCA: right coronary artery.
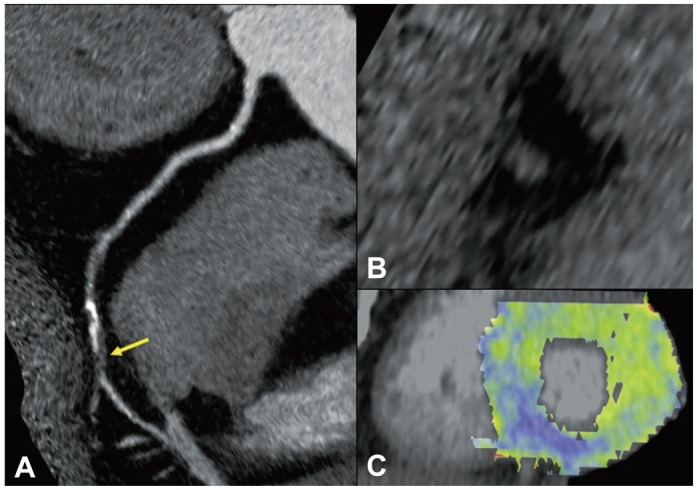
Fig. 2
Extent of coronary artery disease: CCTA results. Multivessel disease (24%) was prevalent in the patients who underwent vascular surgery. Coronary atherosclerosis was found in most of the patients (94%) (History of coronary revascularization and insignificant stenosis in current CCTA has been considered as significantly diseased vessel in this analysis). CCTA: coronary computed tomography angiography.
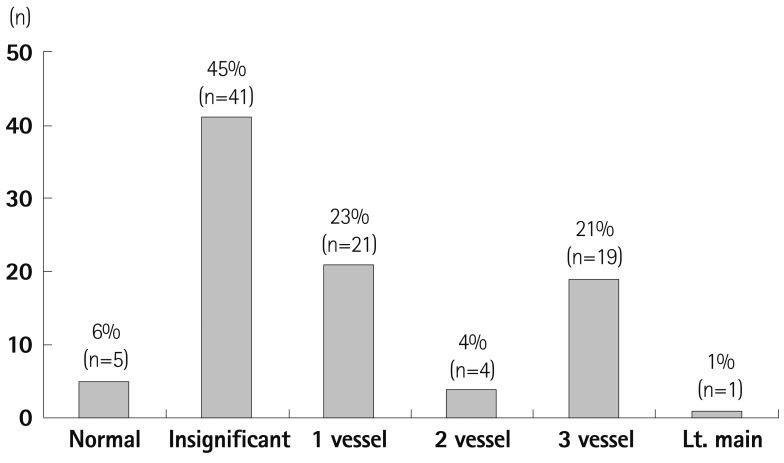
Fig. 3
Result of CCTA and perfusion CT. 94% of the patients had plaques in their coronary arteries and 39% had coronary artery stenosis of more than 50%. However, only a third of the patients with CAD (>50% stenosis) showed a perfusion defect (History of coronary revascularization and insignificant stenosis in current CTA has been considered "insignificant" in this analysis). CCTA: coronary computed tomography angiography, CT: computed tomography, CAD: coronary artery disease.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download