Abstract
Background and Objectives
Screening strategies for aortic aneurysm (AA) according to risk factors and ethnicity are controversial. This study explored the prevalence of AA and determined whether screening is necessary in a population of multiple risk factors.
Subjects and Methods
From June, 2012 to April, 2013, 542 consecutive elderly (≥65 years) male hypertensive patients without a history of AA were prospectively enrolled. After excluding 15 patients (2.8%) with aortic valve surgery, 30 patients (5.5%) with suboptimal computed tomography (CT) images, the remaining 496 patients (age 73±5 years) comprised the study population. Maximal diameters of the thoracic and abdominal aorta were measured using non-contrast CT.
Results
The prevalence of thoracic AA (TAA, diameter ≥40 mm) and abdominal AA (AAA, diameter ≥30 mm) was 36.5% (181/496) and 6.0% (30/496), respectively. In the multivariate logistic regression analysis, determinants for TAA were age {odds ratio (OR) 1.059, 95% confidence interval (CI) 1.018-1.101, p=0.005}, dyslipidemia (OR 0.621, 95% CI 0.418-0.923, p=0.018), body surface area (OR 11.92, 95% CI 2.787-50.97, p=0.001), diastolic blood pressure (OR 1.029, 95% CI 1.009-1.049, p=0.004) and AAA (OR 3.070, 95% CI 1.398-6.754, p=0.005). In contrast, AAA was independently associated with dysplipidemia (OR 2.792, 95% CI 1.091-7.143, p=0.032), current/past smokerfs (OR 4.074, 95% CI 1.160-14.31, p=0.028), and TAA (OR 3.367, 95% CI 1.550-7.313, p=0.002).
Aortic aneurysm (AA) can be life-threatening and aorta size is the best criterion for determining if an intervention is necessary to prevent ruptures, dissections or aneurysm-related deaths.1) However, even large AAs seldom cause symptoms,2)3) although the incidence of aortic disease increases with age.4)5) Therefore, it is important for clinicians to be cautious in their evaluation of patients at risk.
The etiology of AA differs for each segment of the aorta. Aneurysm of the ascending thoracic aorta (ATA) most often results from cystic medial degeneration.2) Atherosclerosis is an infrequent cause of aneurysm of ATA, in contrast to that of the descending thoracic aorta (DTA) and abdominal aorta, where atherosclerosis plays an important role in development of AA.2) Meanwhile, thoracic AAs (TAAs) and abdominal AAs (AAAs) share common risk factors, such as age and hypertension. Therefore, elderly male subjects with hypertension might be at high risk for both TAA and AAA.
Efforts to detect high risk populations for coronary heart disease (CHD) have been widely applied and coronary calcium measurement using non-contrast computed tomography (CT) is the method of choice for assessing cardiovascular risk in asymptomatic subjects.6) Screening for AAA using ultrasound has been recommended in elderly men who are current or past smokers,7) but the screening strategy for TAA in elderly subjects is not well-established, even though it is a fatal disease.8)9) The more widespread applications of cardiac CT and thoracic CT for cardiovascular risk stratification and lung cancer screening have made it possible to measure thoracic aortic diameter using non-contrast CT, resulting in several successful case reports.10)11)
The purpose of the current study was to use non-contrast CT in a prospective manner to explore the prevalence of AAs and determine whether screening for CHD and AAs is necessary in a population with multiple risk factors for CHD in a Korean population.
Five hundreds and two consecutive Korean male hypertensive patients without history of AA were prospectively enrolled from June, 2012 to April, 2013 at the outpatient clinic of Severance Cardiovascular Hospital (Seoul, Korea). Subjects were eligible if they were ≥65 years old and had provided signed informed consent. Of these 542 patients who had done non-contrast CT of whole aorta, 15 patients (2.8%) with a history of aortic valve surgery and 30 patients (5.5%) with a suboptimal CT image were excluded. Most of the suboptimal images were those with errors in raw CT data storage, which caused problems of analysis in the sagittal and coronal planes using reconstructed images. There were 9 cases of respiration-related motion artifacts. The remaining 496 patients comprised the study population. This study was approved by the Institutional Review Board of our institution.
Medical history of hypertension, diabetes and dyslipidemia was obtained from all patients. Height, weight, and blood pressure were measured during their visit. Serum calcium, phosphorus, total cholesterol, low density lipoprotein-cholesterol (LDL-C), high density lipoprotein-cholesterol (HDL-C), triglycerides and serum creatinine levels were measured after a minimum 12-hour fasting period. Hypertension was defined as systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure as ≥90 mm Hg or treatment with antihypertensive agents. Diabetes was defined as treatment with hypoglycemic agents or insulin, or fasting glucose ≥126 mg/dL. Dyslipidemia was defined as any of the following: total cholesterol ≥240 mg/dL, LDL-C ≥130 mg/dL, HDL-C ≤40 mg/dL, triglyceride ≥150 mg/dL or treatment with lipid lowering agents. Smoking history was obtained from patient interviews or medical records.
All examinations were performed using an Aquilion ONE 320-row CT system (Toshiba Medical Systems, Otawara, Japan). Patients were positioned in the supine position on the table. Dual scanograms were used for planning the examination and determining the anatomical range to be covered. Multiple volumes were placed to cover the entire aorta from above the aortic arch to the aortic bifurcation. Patients underwent aorta CT with a prospective electrocardiography-gating wide-volume protocol (from four to six volumes, according to body height). Data were acquired from 40 to 50% of the R-R interval. The CT gantry rotation time was 350 ms. The tube voltage was 120 kV and the effective tube current was adjusted using the adaptive iterative dose reduction three dimensional algorithm. The resulting four to six individual volume data sets were automatically stitched together immediately after reconstruction to generate one CT data set of the whole aorta. All data were reconstructed using a standard soft-tissue and lung kernel (FC43). Images were reconstructed with a slice thickness of 0.5 mm. The CT data sets were reconstructed at 45% of R-R intervals. Mean radiation dose for patients undergoing CT of the whole aorta was 7.0±5.3 mSv.
Maximal ascending aorta diameter (ATAMAX) was measured in the axial plane from just above the aortic root to the aortic arch perpendicular to the aortic axis. Similarly, maximal descending thoracic aorta diameter (DTAMAX) was measured at the DTA distal from the aortic arch to the diaphragm level in the same axis (Fig. 1). ATAMAX and DTAMAX were confirmed in the sagittal and coronal planes using reconstructed images (Fig. 2). Aortic arch diameter was measured in the sagittal plane. Maximal thoracic aorta diameter (TAMAX) was defined as the largest diameter among ATAMAX, DTAMAX and maximal diameter of the aortic arch. Maximal abdominal aortic diameter (AAMAX) was defined as the maximal diameter of the abdominal aorta from the diaphragm to the first slice superior to the aortic bifurcation. TAA was defined as TAMAX ≥40 mm and AAA was defined as AAMAX ≥30 mm.7)12)
Distribution of relevant variables was reported either as a percentage or as the mean±standard deviation. Groups were compared using χ2 statistics for categorical variables. A binary logistic regression analysis was used to identify risk factors associated with TAA and AAA. Variables with a p<0.2 in univariate analysis were included in the multiple logistic regression model. Collinearity among explanatory variables was identified using variance inflation factor (VIF), and highly correlated variables (VIF>5) were excluded. P<0.05 were considered statistically significant.
Patient demographics and clinical characteristics are shown in Table 1. The mean age was 73 years. Diabetes, dyslipidemia and coronary artery disease were observed in 171 patients (34.5%), 318 patients (64.1%) and 256 patients (51.6%), respectively. Three hundred and sixty-four patients (73.4%) had a history of smoking. Mean systolic and diastolic blood pressure were 125.3±14.9 mm Hg and 73.1±14.9 mm Hg. Mean diameter of ATAMAX, DTAMAX, TAMAX, and AAMAX was 38.4±4.0 mm, 28.5±3.0 mm, 38.9±4.0, and 23.0±5.5 mm, respectively. Fig. 3 shows a histogram of TAMAX and AAMAX. The prevalence of TAA and AAA was 36.5% (181/496) and 6.0% (30/496), respectively. There were 16 patients (3.2%) who had TAA and AAA simultaneously. However, prevalence of AA suitable for an indication for intervention according to current guidelines (TAMAX ≥55 mm and AAMAX ≥60 mm)2) was very low in this study. Those who were indicated for intervention were seen only in one case of the TAA group and one case of the AAA group.
Prevalence of TAA was 64.6% in patients with AAA and 40.0% in patients without AAA. Patients with AAA had a higher occurrence of TAA compared with those without AAA (p=0.010) (Fig. 4). Fig. 5 demonstrates difference of prevalence of AA according to history of smoking. There was no significant difference in prevalence of TAA between nonsmokers and those who had a history of smoking or were current smokers. In contrast, prevalence of AAA was significantly higher in current/past smokers compared to non-smokers (p=0.046). Of the 365 current/past smokers, AAA was found in 27 patients (7.4%), but of the 124 non-smokers, AAA was observed in only three patients (2.4%), and there were no nonsmokers with a large AAA (> 40 mm).
Determinants for TAA, AAA and both TAA and AAA are shown in Table 2. In the multivariate logistic regression analysis, determinants for TAA were age {odds ratio (OR) 1.059, 95% confidence interval (CI) 1.018-1.101, p=0.005}, absence of dyslipidemia (OR 0.621, 95% CI 0.418-0.923, p=0.018), body surface area (OR 11.92, 95% CI 2.787-50.97, p=0.001), diastolic blood pressure (OR 1.029, 95% CI 1.009-1.049, p=0.004) and presence of AAA (OR 3.070, 95% CI 1.398-6.754, p=0.005). In contrast, AAA was independently associated with presence of dysplipidemia (OR 2.792, 95% CI 1.091-7.143, p=0.032), current/past smokers (OR 4.074, 95% CI 1.160-14.31, p=0.028) and presence of TAA (OR 3.367, 95% CI 1.550-7.313, p=0.002). However, there were no independent determinants for presence of both TAA and AAA.
Aortic size is critical to key decisions regarding management of AAs.12) This study evaluated the entire aorta diameter, from the thoracic aorta to the abdomen aorta just above the iliac bifurcation, using axial images of non-contrast CT scans in a population with multiple risk factors for both CHD and AA. Prevalence of TAA was significant in this population. The study population included high-risk individuals, but blood pressure and serum lipids were well controlled in most patients, as indicated by blood pressure and laboratory test results. Nevertheless, prevalence of TAA was considerably high and TAA was associated with various clinical factors including older age, larger BSA and higher diastolic blood pressure. Interestingly, TAA was associated with the absence of dyslipidemia, while AAA was significantly associated with the presence of dyslipidemia. One possible explanation might be that the etiology of AAs differs between ascending and descending aorta.1) That is, above the ligamentumarteriosum the disease is non-atherosclerotic, while below the ligamentumarteriosum arteriosclerosis is abundant.1) Therefore, TAA might not be associated with dyslipidemia. Another explanation for the conflicting results might be that the mean values of total cholesterol, LDL-C and HDL-C were almost within normal limits, suggesting the serum lipids were well controlled in most patients. In addition, since the current study was performed at a tertiary referral hospital, many of the patients were taking statins for the primary or secondary prevention for coronary artery disease, not for the treatment of dyslipidemia. Therefore, effects of high lipids on atherosclerosis might be minimized in the studied population and result in conflicting outcomes. Therefore, further studies are needed to clarify the relationship among TAA, AAA, dyslipidemia, serum cholesterol and statin therapy.
Thoracic aortic aneurysm was more prevalent in patients with AAA compared to those without AAA, even after adjusting for multiple confounding factors. However, risk factors for TAA and AAA were different. In addition, more than one-third of the study subjects had a TAA, and it was remarkably more prevalent than AAA among high-risk subjects in the current study, in contrast to previous reports that AAA is more prevalent than TAA.1) TAA prevalence continuously increases,13) and is thought to be related to increased age and hypertension in the general population. The current study focused on elderly patients, which may explain the discrepancy. However, most TAAs had a diameter <50 mm, which is not large enough to indicate a need for immediate intervention. Therefore, the clinical implications of the high prevalence of TAA need further evaluation, since TAAs are known to be an indolent process and to grow very slowly, at approximately 0.1 cm per year.9)14) Future studies should establish a method for aortic screening for TAA and strategies for conservative as well as surgical management in elderly hypertensive patients.
Previous studies consistently demonstrated a relationship between smoking and AAA.11) The present study, which involved a high-risk population of Asian males, found that AAA was independently associated with smoking history in addition to dyslipidemia. Moreover, prevalence of AAA was 2.4% among nonsmokers, which is very low compared to previous studies involving Caucasians.15)16) In addition, none of the nonsmokers had a large AAA (>40 mm). In population-based screening studies in Japan, AAA was rarely encountered17)18) and the prevalence of AAA was also reportedly low in Chinese populations, even in patients with severe coronary artery disease.19) This suggests that race influences AAA prevalence and that Asians generally have a low prevalence of AAA. The present study also reported a low prevalence of AAA in an Asian population and reinforced the impact of smoking on AAA. These results high-light the need to clearly define the usefulness of routine screening for AAA among Asian non-smokers.
The primary limitation of this study is that a CT scan with axial images could not properly evaluate the very proximal portion of the ascending aorta including the aortic root. In addition, even in the sagittal and coronal planes, aortic root diameter could not be accurately measured using non-contrast CT due to the complexity of the structures surrounding the aortic root. Therefore, different imaging modalities are needed to accurately detect aortic root aneurysms. This study was performed in a population of elderly Asian males with hypertension at high risk for cardiovascular disease. Therefore, the results of the current study cannot be applied to females, other ethnicities, or groups with a low-risk of cardiovascular disease.
Figures and Tables
Fig. 1
Measurement of aortic diameter in the axial plane. Ascending and descending thoracic aorta (A) and abdominal aorta (B). ATA: ascending thoracic aorta, DTA: descending thoracic aorta, AA: abdominal aorta.
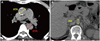
Fig. 2
Reconstructed images of aorta using non-contrast computed tomography. Reconstructed sagittal (A) and coronal (B) images of aorta.
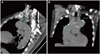
Fig. 3
Histogram of maximal thoracic (A) and abdominal aorta (B). Red lines indicate maximal thoracic aorta of 40 mm and maximal abdominal aorta of 30 mm. TAMAX: maximal thoracic aorta, AAMAX: maximal abdominal aorta.

Fig. 4
Prevalence of thoracic aortic aneurysm in patients with and without abdominal aortic aneurysm. TAA (-), patients without thoracic aortic aneurysm; TAA (+), patients with thoracic aortic aneurysm; AAA (+), patients with abdominal aortic aneurysm; AAA (-), patients without abdominal aortic aneurysm.

Fig. 5
Prevalence of thoracic aortic aneurysm (A) and abdominal aortic aneurysm (B) according to history of smoking. Smoking (+), current or past smoker; Smoking (-), never smoked.

Table 1
Clinical characteristics of the study population

Table 2
Logistic regression analysis of variables as determinants of thoracic aortic aneurysm and abdominal aortic aneurysm

CI: confidential interval, TAA: thoracic aortic aneurysm, AVR: aortic valve replacement, BSA: body surface area, BP: blood pressure, HDL-C: high density lipoprotein-cholesterol, LDL-C: low density lipoprotein-cholesterol, AAA: abdominal aortic aneurysm, ACEi: Angiotensin converting enzyme inhibitor, ARB: aldosteron receptor blocker, CCB: calcium channel blocker
Acknowledgments
This research was supported by Leading Foreign Research Institute Recruitment Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (MSIP) (No. 2012027176).
References
1. Elefteriades JA, Farkas EA. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J Am Coll Cardiol. 2010; 55:841–857.
2. Isselbacher EM. Thoracic and abdominal aortic aneurysms. Circulation. 2005; 111:816–828.
3. Lindholt JS, Henneberg EW, Fasting H, Juul S. Mass or high-risk screening for abdominal aortic aneurysm. Br J Surg. 1997; 84:40–42.
4. Clouse WD, Hallett JW Jr, Schaff HV, et al. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc. 2004; 79:176–180.
5. Acosta S, Ogren M, Bengtsson H, Bergqvist D, Lindblad B, Zdanowski Z. Increasing incidence of ruptured abdominal aortic aneurysm: a population-based study. J Vasc Surg. 2006; 44:237–243.
6. Taylor AJ, Cerqueira M, Hodgson JM, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2010; 56:1864–1894.
7. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; Trans-Atlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006; 113:e463–e654.
8. Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg. 2002; 74:S1877–S1880. discussion S1892-8.
9. Davies RR, Goldstein LJ, Coady MA, et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg. 2002; 73:17–27. discussion 27-8.
10. Wolak A, Gransar H, Thomson LE, et al. Aortic size assessment by noncontrast cardiac computed tomography: normal limits by age, gender, and body surface area. JACC Cardiovasc Imaging. 2008; 1:200–209.
11. Mao SS, Ahmadi N, Shah B, et al. Normal thoracic aorta diameter on cardiac computed tomography in healthy asymptomatic adults: impact of age and gender. Acad Radiol. 2008; 15:827–834.
12. Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: executive summary. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Catheter Cardiovasc Interv. 2010; 76:E43–E86.
13. Olsson C, Thelin S, Ståhle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation. 2006; 114:2611–2618.
14. Rizzo JA, Coady MA, Elefteriades JA. Procedures for estimating growth rates in thoracic aortic aneurysms. J Clin Epidemiol. 1998; 51:747–754.
15. Jamrozik K, Norman PE, Spencer CA, et al. Screening for abdominal aortic aneurysm: lessons from a population-based study. Med J Aust. 2000; 173:345–350.
16. Singh K, Bønaa KH, Jacobsen BK, Bjørk L, Solberg S. Prevalence of and risk factors for abdominal aortic aneurysms in a population-based study: The Tromsø Study. Am J Epidemiol. 2001; 154:236–244.
17. Takei H, Ishikawa S, Otaki A, et al. Screening for abdominal aortic aneurysm and occlusive peripheral vascular disease in Japanese residents. Surg Today. 1995; 25:608–611.
18. Adachi K, Iwasawa T, Ono T. Screening for abdominal aortic aneurysms during a basic medical checkup in residents of a Japanese rural community. Surg Today. 2000; 30:594–599.
19. Poon JT, Cheng SW, Wong JS, Ting AC. Prevalence of abdominal aortic aneurysm in Chinese patients with severe coronary artery disease. ANZ J Surg. 2010; 80:630–633.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download