Abstract
Background and Objectives
An increase in intracellular calcium concentration due to loss of Ca2+ homeostasis triggers arrhythmia or cardiac cell death in the heart. Paracrine factors released from stem cells have beneficial cardioprotective effects. However, the mechanism of modulation of Ca2+ homeostasis by paracrine factors in ischemic myocardium remains unclear.
Materials and Methods
We isolated rat bone marrow-derived mesenchymal stem cells (MSCs), and prepared paracrine media (PM) from MSCs under hypoxic or normoxic conditions (hypoxic PM and normoxic PM). We induced rat myocardial infarction by left anterior descending ligation for 1 hour, and treated PM into the border region of infarcted myocardium (n=6/group) to identify the alteration in calcium-regulated proteins. We isolated and stained the heart tissue with specific calcium-related antibodies after 11 days.
Results
The hypoxic PM treatment increased Ca2+-related proteins such as L-type Ca2+ channel, sarcoplasmic reticulum Ca2+ ATPase, Na+/K+ ATPase, and calmodulin, whereas the normoxic PM treatment increased those proteins only slightly. The sodium-calcium exchanger was significantly reduced by hypoxic PM treatment, compared to moderate suppression by the normoxic PM treatment.
Cardiac necrosis and apoptosis occurs during myocardial infarction (MI) due to oxidative stress and calcium accumulation.1) Accumulation of intracellular calcium triggers arrhythmia and the proapoptotic process.2) Calcium cycling plays a key role in contraction of the heart by excitation-contraction coupling within the cardiac muscle cells.3) Therefore, loss of calcium homeostasis, such as an increase in intracellular calcium, is important to trigger the process leading to cardiac cell death.4)
Stem cell therapy can potentially provide beneficial paracrine factors for cellular repair to protect damaged myocardium and to restore or regenerate injured myocardium.5) Mesenchymal stem cells (MSCs) have been proposed as a potential source for MI cell therapy.3)4) Indeed, several researchers have reported that implanting MSCs into infarcted hearts reduces post-infarction ventricular remodeling and infarct size and improves left ventricular function.6)7) We have previously reported that bone marrow-derived MSCs engrafted in the border zone reduce infarct size.8) Despite the promising application of MSCs, proarrhythmic potentials due to the cellular heterogeneity between stem cells and cardiomyocytes in the transplanted region may be an issue.9) However, paracrine molecules secreted by MSCs increase oxygen and nutrient tissue levels, providing a rich environment for grafted MSCs and endogenous cardiomyocyte proliferation.10-12) This process may contribute largely to the antiarrhythmic effect. We found that paracrine factors released from MSCs under hypoxia contribute to alleviate ectopic focal activity in the border zone of which the mechanism is related to abnormal calcium cycling. Furthermore, we observed that the paracrine media (PM) generated under a hypoxic condition reduces intra-cellular calcium concentrations in hypoxic cardiomyocytes and restores mRNA expression of calcium-related proteins in the infarct region border.13)
In this study, we investigated whether paracrine factors affect expression of calcium-related proteins in post-infarcted heart.
Rat bone marrow MSCs were harvested from 1-month-old male Sprague-Dawley rats. After anesthesia with ketamine (10 mg/kg) and xylazine (5 mg/kg), the tibia and femur were dissected, and whole bone marrow was flushed by means of an 18-gauge needle and 10 mL syringe loaded with Dulbecco's Modified Eagle's Medium (DMEM)-Low Glucose supplemented with 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA, USA). The flushed medium was centrifuged at 1600 rpm for 5 minutes and resuspended in serum-supplemented medium. Next, the medium was loaded onto 4 mL of Ficoll-Paque PLUS (GE Healthcare Life Science, Parsippany, NJ, USA) density gradient centrifugation medium per 3 rats at 1600 rpm for 30 minutes. Mononuclear cells recovered from the middle interface of the Ficoll-separated bone marrow and blood were washed twice and resuspended in PBS. MSCs were incubated in fresh DMEM by adding 10% FBS and 100 U/mL penicillin/streptomycin. Cells were maintained in a 37℃ CO2 incubator for 10 days, with fresh medium changes every 3 days.
Second passage MSCs that were 90% confluent (1×106 cells) were incubated under hypoxia or normoxia in serum-free DMEM for 12 hours. The hypoxic condition was created by incubating MSCs at 37℃ in an anaerobic system (Technomart Inc., Seoul, Korea) with a 5% CO2, 5% H2 and 85% N2 atmosphere, and a chamber oxygen level of 0.5%. Hypoxic PM or normoxic PM were collected for injection into ischemic myocardium, were centrifuged to remove cell debris, and concentrated by VIVASPIN6 (Vivascience Ltd.). The medium was continuously centrifuged a 1000×g for 30 minutes at 4℃ using an ultracentrifuge.
All animal experimental procedures were approved by the Committee for Care and Use of Laboratory Animals, Yonsei University College of Medicine, and performed in accordance with the Guidelines and Regulations for Animal Care. MI was produced in male Sprague-Dawley rats (250-300 g) by left anterior descending (LAD) coronary artery ligation, as described above. After anesthesia with ketamine (100 mg/kg) and xylazine (5 mg/kg), the hearts were excised by opening the chest at the third and fourth ribs. The left coronary artery was ligated 2-3 mm from its origin with 5-0 Prolene suture (Ethicon, Summit, NJ, USA). After a 1 hour occlusion, the infarcted heart was reperfused and saline, hypoxic PM, normoxic PM, or MSCs were injected into the border region. The PM of MSCs or saline was enriched to 100 µL and injected into the infarct border region at three different sites using a syringe with a 30-gauge needle. Cells (1×106 cells) were suspended in 10 µL of serum-free medium and injected into the border in the same way for MSC transplantation. Rats were killed 11 days after injection of hypoxic PM, normoxic PM, or MSCs (n=6, each).
The heart was perfused and fixed with 10% (v/v) neutral buffered formaldehyde for 24 hours, and then transversely sectioned into four comparably thick paraffin embedded sections. Five µm sections were mounted on gelatin-coated glass slides for different staining.
We used 3,3'-diaminobenzidine (DAB, Vector Laboratories, Burlingame, CA, USA) or antibody-tagged fluorescence to detect the expression of calcium-related proteins. After de-paraffinization and rehydration, the sample was rinsed with PBS. Antigen retrieval was performed with 10 mM sodium citrate (pH 6.0) in a microwave for 10 minutes. The sections were incubated in 3% H2O2 to quench endogenous peroxidase activity. The samples were blocked in 2.5% normal horse serum diluted in PBS and then incubated with primary antibodies {sarcoplasmic reticulum Ca2+ ATPase (SERCA2a); L-type Ca2+ channel (LTCC); sodium-calcium exchanger (NCX); Santa Cruz Biotechnology, Santa Cruz, CA, USA; Na+/K+ ATPase and calmodulin, Abcam, Cambridge, MA, USA} overnight at 4℃. The tissue slides were biotinylated with a pan-specific universal secondary antibody (R.T.U. VECTASTAIN Universal Quick Kit; Vector Laboratories). Tissue stain was visualized by adding DAB for 5 minutes, and was stained with methyl green to assess nuclei and cytologic details. A coverslip was placed on each section, and the sections were observed by light microscopy. The samples were incubated with fluorescein isothiocyanate-conjugated or Texas red-conjugated antibodies for fluorescence staining (Jackson ImmunoResearch, West Grove, PA, USA) for 1 hour. All images were produced using an excitation filter under reflected light fluorescence microscopy (LSM 700, Carl Zeiss, Oberkochen, Germany).
Altered expression of calcium regulatory ion channels and proteins in the border zone contributed to altered conduction, which we observed previously by optical mapping.14) Levels of intracellular Ca2+ and Na+ play a key role in maintenance of physiological cycling of action potentials and excitation-contraction coupling.15) We observed a decrease in the expression of LTCC, SERCA2a, and Na+/K+ ATPase in the sham group, whereas marked increases in these were observed in the border zone of the hypoxic PM group. However, injection of normoxic PM or MSCs into the border zone only moderately increased expression of LTCC, SERCA2a, and Na+/K+ ATPase (Figs. 1 and 2). We also examined expression of the calcium-binding protein, calmodulin, in the border zone, which was significantly restored in the hypoxic PM-treated group and moderately restored in the normoxic PM and MSC groups (Fig. 1). These findings demonstrate that hypoxic PM may exert a positive effect on Ca2+ homeostasis in the border zone of infarcted myocardium. In addition, MSCs injected into the ischemic border zone and their release could result in the secretion of paracrine molecules similar to components of normoxic PM.
Sodium-calcium exchanger is essential for Ca2+ flux to remove Ca2+ from the cell during excitation-contraction coupling but it may trigger arrhythmia after depolarization due to the electrogenic effect.16) Indeed, over-expression and enhanced activity of NCX increase the propensity for arrhythmia.17) NCX expression in the border zone decreased remarkably following treatment of hypoxic PM, compared to that of sham, demonstrating elevated NCX expression (Fig. 1). Treatment of normoxic PM and MSCs reduced NCX fluorescence intensity slightly, suggesting a modest effect on NCX expression.
Stem cells have been proposed as a potential cell therapy for acute MI as they can undergo cardiomyogenic differentiation.3-5) However, the mechanisms underlying their therapeutic effects have not been clearly defined, and low viability of stem cells transplanted into the myocardium may limit cardiac regeneration.18) Furthermore, a heterogeneous feature of stem cells coupled to naive cardiomyocytes may lead to increased arrhythmogeneity.9)19) Some studies have shown that injection of MSC culture media is a superior strategy to rescue tissue damage resulting from ischemic insults.20) In Gnecchi's report, conditioned media from hypoxic cultured Akt-overexpressed MSCs mediated cardiac protection and functional improvement.21) We previously examined the antiarrhythmic potential of PM from hypoxic MSCs and found that hypoxic PM decreases intracellular Ca2+ concentration with attenuation of Fluo-4 intensity in an in vitro study.13)
In this study, we found that hypoxic PM dramatically modulated the ion channels and proteins related to intracellular Ca2+ levels in infarcted myocardium. PM released from MSCs under the normoxic condition had an intermediate effect on the expression levels of Ca2+ regulating proteins. Indeed, ischemia affects pH regulating systems including the Na+/H+ exchanger and suppresses ATP-driven Na+-K+ pumps, facilitating reverse activity of NCX and, consequentially, contributing to intracellular Ca2+ overload.22)23) Therefore, rescue from Ca2+ overload is regarded as a key point during recovery from hypoxic myocardial damage.
Protein levels of NCX, which plays a role in trans-sarcolemmal calcium extrusion, increase during heart failure24) and play a major role in occurrence of triggered activity such as delayed after-depolarization.16) Sarcolemmal NCX protein levels increased in the MI group, but the hypoxic PM led to a decrease in expression. LTCC is critically involved in excitation-contraction coupling, whereby membrane depolarization activates (open) LTCC, allowing extracellular Ca2+ entry. The amount of LTCC decreased significantly in the MI group, but the hypoxic PM treatment restored the levels to normal. In addition, the Ca2+-handling protein, calmodulin, is an important regulator of ion homeostasis.25) Our results show that the hypoxic PM treatment restored calmodulin expression, as supported by the previous results; calmodulin expression decreases in human ischemic heart disease, and calmodulin decreased in hypoxic myocytes.25) SERCA2a, which functions in transport of calcium from the cytosol to the sarcoplasmic reticulum lumen, decreases in ischemic heart.26) In the current study, the analysis of SERCA2a expression patterns demonstrated that hypoxic PM was rescued from down-regulated expression of SERCA2a in the border. Na+/K+ ATPase provides energy for calcium transfer at the external membrane level, thereby involving Ca2+ homeostasis. In our study, the decreased Na+/K+ ATPase expression in the MI group was restored to normal by the hypoxic PM treatment. Our data demonstrate no significant effects of normoxic PM on the expression levels of Na+/K+ ATPase, SERCA2a, or calmodulin in the border of infarcted myocardium and it had less of an effect on NCX and LTCC than that of the hypoxic PM.
Our results suggest that the expression of Ca2+-related proteins restored by paracrine factors from hypoxic MSCs may lead to suppress abnormal focal arrhythmia and prevent apoptosis, as shown in our previous study.13)
The current study had several limitations. First, we did not identify the expression levels of Ca2+-related proteins except SERCA2a. Instead, we investigated mRNA expression levels of LTCC, NCX, Na+/K+ ATPase, SERCA2a, and calmodulin by reverse transcription polymerase chain reaction and found that hypoxic paracrine molecules restored mRNA expression levels of these proteins.13) Second, we observed only the early phase of MI following LAD ligation. Because there is a lack of appropriate therapeutic tools to prevent fatal arrhythmic events during this period, we focused on the short period of early MI to evaluate the possibility of hypoxic paracrine molecules as a potential drug to target the early phase of MI.13)27) Indeed, we found that paracrine molecules secreted from hypoxic MSCs may be a potential candidate drug to suppress life-threatening ventricular arrhythmias during the early phase of MI,13) by restoring expression of Ca2+-related proteins in the border zone.
Figures and Tables
Fig. 1
Alteration of calcium-related proteins regulating excitation-contraction coupling. LTCC, NCX, and calmodulin were detected by DAB staining. Sections were counterstained with methyl green. All sections were observed by virtual microscopy (original magnification: 100×, scale bars: 200 µm). LTCC: L-type Ca2+ channel, NCX: sodium-calcium exchanger, DAB: 3,3-diamino benzidine, PM: paracrine media, MSCs: mesenchymal stem cells.
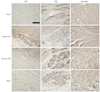
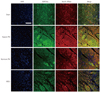
Fig. 2
Effect of hypoxic PM during repolarization of infarcted myocardium. Infarcted myocardium treated with PM or MSCs was analyzed for calcium-(SERCA2a, green) and potassium-related protein (Na+/K+ ATPase, red) by immunofluorescence staining. Nuclei were stained with DAPI. All sections were observed by confocal microscopy (original magnification: 100×, scale bars: 200 µm). PM: paracrine media, MSCs: mesenchymal stem cells, SERCA2a: sarcoplasmic reticulum Ca2+ ATPase, DAPI: 4',6-diamidino-2-phenylindole, dihydrochloride.
Acknowledgments
This research was supported by a grant from the Korean Society of Cardiology (2007) and the Basic Science Research Program through the National Research Foundation of Korea (2011-0014595) funded by the Ministry of Education, Science, Republic of Korea.
References
1. Webster KA, Graham RM, Thompson JW, et al. Redox stress and the contributions of BH3-only proteins to infarction. Antioxid Redox Signal. 2006; 8:1667–1676.
2. Orrenius S, McConkey DJ, Bellomo G, Nicotera P. Role of Ca2+ in toxic cell killing. Trends Pharmacol Sci. 1989; 10:281–285.
3. Mathur A, Martin JF. Stem cells and repair of the heart. Lancet. 2004; 364:183–192.
4. Couzin J, Vogel G. Cell therapy. Renovating the heart. Science. 2004; 304:192–194.
5. Orlic D, Hill JM, Arai AE. Stem cells for myocardial regeneration. Circ Res. 2002; 91:1092–1102.
6. Min JY, Sullivan MF, Yang Y, et al. Significant improvement of heart function by cotransplantation of human mesenchymal stem cells and fetal cardiomyocytes in postinfarcted pigs. Ann Thorac Surg. 2002; 74:1568–1575.
7. Mangi AA, Noiseux N, Kong D, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003; 9:1195–1201.
8. Song H, Chang W, Lim S, et al. Tissue transglutaminase is essential for integrin-mediated survival of bone marrow-derived mesenchymal stem cells. Stem Cells. 2007; 25:1431–1438.
9. Chang MG, Tung L, Sekar RB, et al. Proarrhythmic potential of mesenchymal stem cell transplantation revealed in an in vitro coculture model. Circulation. 2006; 113:1832–1841.
10. Nagaya N, Fujii T, Iwase T, et al. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am J Physiol Heart Circ Physiol. 2004; 287:H2670–H2676.
11. Miyahara Y, Nagaya N, Kataoka M, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006; 12:459–465.
12. Nagaya N, Kangawa K, Itoh T, et al. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005; 112:1128–1135.
13. Hwang HJ, Chang W, Song BW, et al. Antiarrhythmic potential of mesenchymal stem cell is modulated by hypoxic environment. J Am Coll Cardiol. 2012; 60:1698–1706.
14. Decker KF, Rudy Y. Ionic mechanisms of electrophysiological heterogeneity and conduction block in the infarct border zone. Am J Physiol Heart Circ Physiol. 2010; 299:H1588–H1597.
15. Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008; 70:23–49.
16. Sipido KR, Bito V, Antoons G, Volders PG, Vos MA. Na/Ca exchange and cardiac ventricular arrhythmias. Ann N Y Acad Sci. 2007; 1099:339–348.
17. Venetucci LA, Trafford AW, O'Neill SC, Eisner DA. Na/Ca exchange: regulator of intracellular calcium and source of arrhythmias in the heart. Ann N Y Acad Sci. 2007; 1099:315–325.
18. Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002; 105:93–98.
19. Beeres SL, Atsma DE, van der Laarse A, et al. Human adult bone marrow mesenchymal stem cells repair experimental conduction block in rat cardiomyocyte cultures. J Am Coll Cardiol. 2005; 46:1943–1952.
20. Berry MF, Engler AJ, Woo YJ, et al. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol. 2006; 290:H2196–H2203.
21. Gnecchi M, He H, Noiseux N, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006; 20:661–669.
22. Ostadal P, Elmoselhi AB, Zdobnicka I, Lukas A, Chapman D, Dhalla NS. Ischemia-reperfusion alters gene expression of Na+-K+ ATPase isoforms in rat heart. Biochem Biophys Res Commun. 2003; 306:457–462.
23. Ehrlich BE, Kaftan E, Bezprozvannaya S, Bezprozvanny I. The pharmacology of intracellular Ca(2+)-release channels. Trends Pharmacol Sci. 1994; 15:145–149.
24. Leszek P, Szperl M, Klisiewicz A, et al. Alteration of myocardial sarcoplasmic reticulum Ca2+-ATPase and Na+-Ca2+ exchanger expression in human left ventricular volume overload. Eur J Heart Fail. 2007; 9:579–586.
25. Jeck CD, Zimmermann R, Schaper J, Schaper W. Decreased expression of calmodulin mRNA in human end-stage heart failure. J Mol Cell Cardiol. 1994; 26:99–107.
26. Talukder MA, Kalyanasundaram A, Zuo L, et al. Is reduced SERCA2a expression detrimental or beneficial to postischemic cardiac function and injury? Evidence from heterozygous SERCA2a knockout mice. Am J Physiol Heart Circ Physiol. 2008; 294:H1426–H1434.
27. Solomon SD, Zelenkofske S, McMurray JJ, et al. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N Engl J Med. 2005; 352:2581–2588.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download