Abstract
Background and Objectives
We evaluated the two-year clinical outcomes in patients with angiographically intermediate lesions according to the plaque burden and treatment strategy.
Subjects and Methods
We prospectively enrolled patients with angiographically intermediate lesions (diameter stenosis 30-70%) with an intravascular ultrasound (IVUS) minimum lumen area (MLA) <4 mm2 with 50-70% plaque burden of 16 Korean percutaneous coronary intervention centers. Patients were divided into medical therapy group (n=85) and zotarolimus-eluting stent group (ZES; Resolute) group (n=74). We evaluated the incidences of two-year major adverse cardiovascular events (MACE).
Results
A two-year clinical follow-up was completed in 143 patients and MACE occurred in 12 patients. There were no significant differences in the incidences of death (1.3% vs. 3.0%, p=0.471), target vessel-related non-fatal myocardial infarction (0.0% vs. 0.0%, p=1.000) and target vessel revascularizations (7.8% vs. 4.5%, p=0.425) between medical and ZES groups. Independent predictors of two-year MACE included acute myocardial infarction {odds ratio (OR)=2.87; 95% confidence interval (CI) 1.43-6.12, p=0.014}, diabetes mellitus (OR=2.46; 95% CI 1.24-5.56, p=0.028) and non-statin therapy (OR=2.32; 95% CI 1.18-5.24, p=0.034).
Clinical decision making and the management of intermediate coronary stenosis, defined by a diameter stenosis of 30% to 70% continues to be a therapeutic dilemma for cardiologists.1)2) Intravascular ultrasound (IVUS) examination and functional assessment of coronary stenosis have been used to define the severity of such lesions and to designate patients to the most appropriate therapy.3)4)5)6) Although optimal IVUS criteria for predicting functional significance of intermediate coronary lesions are variable according to individual studies which have been performed recently,7)8)9) the cut-off value of IVUS minimum lumen area (MLA) 4 mm2 is still being used for the prediction of future clinical events.
Currently, no relevant data are available for the comparison between medical therapy and drug-eluting stent for intermediate lesions, that are particularly common in Asian patients.10)11) We hypothesized that medical therapy is not inferior to drug-eluting stent for the treatment of the intermediate lesions with IVUS MLA <4 mm2 with 50-70% of plaque burden. Therefore, the purpose of the present study was to compare the optimal medical therapy and the drug-eluting stent for the long-term clinical outcome in patients with angiographically intermediate lesions with IVUS MLA <4 mm2 with 50-70% of plaque burden.
The investigators of the Korean MultIceNter TrIal on Long-Term Clinical Outcome According to the Plaque Burden and Treatment Strategy in Lesions with MinimUm Lumen ARea LEss Than 4 mm2 Using Intravascular Ultrasound (MINIATURE) study were selected of 16 major percutaneous coronary intervention centers in Korea (http://miniaturestudy.or.kr). The study algorithm is shown in Fig. 1. Patients were eligible for this study if they were at least 18 years of age and showed intermediate coronary artery stenosis (diameter stenosis 30-70%) with IVUS MLA <4 mm2 with 50-70% of plaque burden on coronary angiogram. Culprit lesion in silent ischemia or stable angina was defined as the coronary lesion whose diameter stenosis by QCA was greatest in a patient with multi-vessel disease. Culprit lesion was defined as the site of acute coronary occlusions or, for non-occluded arteries, as the site of greatest narrowing within an angiographically significant stenosis corresponding to the electrocardiographic changes in patients with acute coronary syndrome. Target lesions enrolled in the present study were non-culprit lesions that had not been treated with percutaneous coronary intervention in patients with acute coronary syndrome. We initially treated the culprit lesions first and observed the non-culprit lesions in other arteries in patients with acute coronary syndrome. Major exclusion criteria for the study enrollment were a known hypersensitivity or contraindication to heparin, anti-platelet agents, zotarolimus or contrast media; systemic (intravenous) use of zotarolimus within 12 months, severe left ventricular systolic dysfunction (left ventricular ejection fraction <25%); cardiogenic shock and left main coronary artery lesion. One hundred fifty nine patients who underwent IVUS examination for angiographically intermediate lesions with IVUS MLA <4 mm2 with 50-70% of plaque burden (Fig. 2) were prospectively enrolled between November 2008 and May 2010. Zotarolimus-eluting stent (ZES; Resolute, Medtronic, Minneapolis, MN, USA) was chosen as the only type of stent in the present study and patients were randomized into medical therapy group (n=85) or ZES group (n=74). We enrolled the non-culprit lesion if the patient had a multi-vessel disease and ZES was deployed for the culprit lesion in the culprit vessel and an intermediate lesion was located at the non-culprit vessel. The data were analyzed by quantitative coronary angiography (QCA) and IVUS core laboratory (Chonnam National University Hospital). Sixteen patients (8 patients in each group) were lost to follow-up during two years of clinical follow-up. Finally 143 patients were clinically followed up for two years. The protocol was approved by the ethics committee of each participating medical center and the patients granted their consent to participate in the study after the index procedure.
Coronary angiogram was analyzed by a validated QCA system (Philips H5000 or Allura DCI program, Philips Medical Systems, Amsterdam, North Holland, the Netherlands). Reference diameter and minimal lumen diameter were measured in diastolic frames of orthogonal projections with the outer diameter of the contrast-filled catheter as the calibration standard. Perfusion was evaluated according to the Thrombolysis in Myocardial Infarction (TIMI) criteria.12)
All IVUS examinations were performed after intracoronary administration of 300 µg nitroglycerin using a commercially available IVUS system in each participating hospital. The IVUS catheter was advanced distal to the target lesion and imaging was performed retrograde to the aorto-ostial junction at an automatic pullback speed of 0.5 mm/sec.
Qualitative analysis was performed according to the American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies.13) We measured the external elastic membrane (EEM) and lumen cross-sectional area (CSA) using planimetry software (Echoplaque 3.0, INDEC Systems Inc., Santa Clara, CA, USA). The plaque plus media (P&M) CSA was calculated as EEM CSA minus lumen CSA and the plaque burden was calculated as P&M CSA divided by EEM CSA. Proximal and distal references were the single slices with the largest lumen and smallest plaque CSAs within 5 mm proximally and distally, but before any large side branch.
Hypoechoic plaque was less bright compared with the reference adventitia. Hyperechoic noncalcified plaque was as bright as or brighter than the reference adventitia without acoustic shadowing. Hyperechoic calcified plaque was hyperechoic with shadowing. A calcified lesion contained >90° of circumferential lesion calcium. The plaque was classified as mixed if there was no dominant plaque composition.
Hospital records of all patients were reviewed to obtain information on clinical demographics and medical history. Follow-up information was obtained through review of hospital charts, telephone interviews and the interventional database of each participating center. Major adverse cardiovascular event (MACE) included death, target vessel-related non-fatal myocardial infarction, target lesion and target vessel revascularization (TLR and TVR). A target-vessel related non-fatal myocardial infarction was defined as ischemic symptoms associated with cardiac enzyme elevation ≥3 times the upper limit of normal value. TLR was defined as percutaneous coronary intervention of the lesion site including 5-mm proximal or distal to the lesion in medical therapy group and as repeated revascularization of a lesion anywhere within the stent or the 5-mm borders proximal or distal to the stent in ZES group. TVR was defined as any revascularization of the target lesion or any segment of the epicardial coronary artery containing the target lesion.
The Statistical Package for the Social Sciences (SPSS) for Windows version 15.0 (SPSS Inc., Chicago, IL, USA) was used for all analyses. Continuous variables were presented as the mean value±1 SD; comparisons were conducted by Student's t-test or nonparametric Wilcoxon test if normality assumption was violated. Discrete variables were presented as percentages and relative frequencies; comparisons were conducted by chi-square statistics or Fisher's exact test as appropriate. Multivariate analysis was performed to determine the independent predictors of two-year MACE. A p<0.05 was considered statistically significant.
The baseline characteristics, coronary angiography and IVUS findings are summarized in Table 1. There were no significant differences in baseline characteristics, coronary angiography and IVUS findings in both groups. About one fifth of the patients had myocardial infarction without statistical significance between medical therapy and ZES group. More than two thirds of the target vessel was the left anterior descending artery, 94.3% of the patients had TIMI flow grade 3, the minimal lumen diameter was 1.12±0.17 mm and the diameter stenosis was 63±5%. The MLA was 3.3±1.2 mm2, the plaque burden at the MLA site was 66±4% and the lesion length was 14.7±7.0 mm.
Medications and MACE at two-year follow-up are summarized on Table 2. Clopidogrel was more frequently used in ZES group than in medical therapy group and the use of other medications were not different between both groups at two years follow-up. The follow-up low-density lipoprotein cholesterol level was 83±36 mg/dL in medical therapy group and 80±28 mg/dL in ZES group.
Major adverse cardiovascular event occurred in 4.7% in medical therapy group and in 5.4% of cases in ZES group during one year of follow-up and in 9.1% in medical therapy group and in 7.5% of cases in ZES group during two years of follow-up. Four MACE (including one death, three TLRs and three TVRs) in the medical therapy group and four MACE (including two deaths, two TLRs and two TVRs) in the ZES group occurred during one year of follow-up. Sixteen patients were lost to follow-up during the two years of follow-up. A two-year follow-up was completed for 77 patients in medical therapy group and for 66 patients in ZES group. Seven MACE (including one death, six TLRs and six TVRs) in medical therapy group and five MACE (two deaths, two TLRs and three TVRs) in ZES group occurred during two years of follow-up. There was no target-vessel related non-fatal myocardial infarction in both groups and no stent thrombosis occurred in the ZES group. There were no significant differences in the occurrence of MACE between medical therapy group and ZES group after two years.
The prevalence of myocardial infarction (58.3% vs. 15.3%, p<0.001) and diabetes mellitus (58.3% vs. 29.0%, p=0.024) was significantly higher in patients with MACE compared with those without MACE. Glucose, creatine kinase-myocardial band, cardiac specific troponin-I and N-terminal pro-B type natriuretic peptide were significantly higher in patients with MACE compared with those without MACE. At two-year follow up, statins were used less frequently in patients with MACE compared with those without MACE (50.0% vs. 77.9%. p=0.032).
There were no significant differences in target vessel, reference diameter, minimal lumen diameter and diameter stenosis between patients with MACE and those without MACE. However, a TIMI flow grade 3 was less frequently observed in patients with MACE compared with those without MACE (75.0% vs. 96.2%, p<0.001).
There were no significant differences in MLA and plaque burden at MLA site and plaque morphology between patients with MACE compared with those without MACE. The plaque burden at the distal reference segment was significantly greater (32±8% vs. 26±7%, p=0.019) and IVUS lesion was significantly longer (19.4±17.3 mm vs. 14.7±9.8 mm, p=0.010) in patients with MACE compared with those without MACE.
Multivariate analysis was performed to identify independent predictors of two-year MACE (Table 3). The following variables were tested (with p<0.1 in univariate analysis): acute myocardial infarction, diabetes mellitus, smoking, N-terminal pro-B type natriuretic peptide, non-statin use, TIMI flow grade, plaque burden at distal reference segment and IVUS lesion length. Independent predictors of two-year MACE included acute myocardial infarction {odds ratio (OR)=2.87; 95% confidence interval (CI) 1.43-6.12, p=0.014}, diabetes mellitus (OR=2.46; 95% CI 1.24-5.56, p=0.028) and non-statin therapy (OR=2.32; 95% CI 1.18-5.24, p=0.034).
The present study demonstrated that 1) MACE occurred in 9.1% of medical therapy group and in 7.5% of ZES group during two years of follow-up, 2) the prevalence of myocardial infarction and diabetes mellitus was significantly higher in patients with two-year MACE compared with those without two-year MACE, 3) statins were less frequently used in patients with two-year MACE compared with those without two-year MACE, and 4) myocardial infarction, diabetes mellitus and non-statin therapy were the independent predictors of MACE in patients with angiographically intermediate lesions with IVUS MLA <4 mm2 with 50-70% of plaque burden during two-year follow-up.
Intermediate coronary lesion remains an unresolved problem for interventional cardiologists treating patients with coronary artery disease. Previous study suggested that IVUS MLA of <4 mm2 was found to be the threshold for flow-limiting stenosis.3) However, this criterion is only applicable to lesions located at proximal epicardial coronary arteries with a reference segment diameter >3 mm, limiting the use of IVUS-derived anatomic criteria to define the functional significance of a subset of lesions. Furthermore, lower cut-off value of MLA has been recently suggested to predict functionally significant coronary stenosis.7)8)9) Although optimal IVUS criteria for predicting functional significance of intermediate coronary lesions are variable, The Providing Regional Observations to Study Predictors of Events in the Coronary Tree (PROSPECT) study14) showed non-culprit lesions associated with recurrent events were more likely than those not associated with recurrent events to be characterized by MLA <4.0 mm2 or plaque burden >70% or to be classified on the basis of radiofrequency IVUS as thin-cap fibroatheromas.
The present study compared the optimal medical therapy and ZES for the long-term clinical outcome in patients with angiographically intermediate lesions with IVUS MLA <4 mm2 with 50-70% of plaque burden. Similar to the PROSPECT study, MACE occurred in 9.1% in medical therapy group and in 7.5% in ZES group during two years of follow-up.14) There was no target vessel-related non-fatal myocardial infarction in both medical therapy group and ZES group and there was no stent thrombosis (early, late and very late stent thrombosis) in ZES group during two years of follow-up. Medical therapy is not inferior to treat patients with intermediate stenosis with MLA <4 mm2 with 50-70% of plaque burden if compared with ZES. Optimal medical therapy as well as ZES implantation is a good option for the treatment of angiographically intermediate lesions.
In the present study, myocardial infarction, diabetes mellitus and non-statin therapy were the independent predictors of MACE during two years of follow-up. In the present study, of 12 MACEs were nine TVR-related and three were deaths. The risk of plaque progression is high in patients with AMI, in diabetic patients and in patients without statins. Patients with acute myocardial infarction have more vulnerable plaque components in their culprit lesion in addition to their non-culprit lesion. The occurrence of MACE during follow-up was equally attributable to the recurrence at the site of culprit lesions and to non-culprit lesions and most of nonculprit lesions had thin-cap fibroatheromas in the PROSPECT study.14) Therefore, a non-culprit lesion with intermediate stenosis in patients with acute myocardial infarction has the potential to develop plaque progression and to provoke future events. A rapid progression of atherosclerosis can be seen in diabetic patients.15) An impaired glycemic homeostasis has direct influence on the propagation of atherosclerotic plaque16) and plaque regression and stabilization are attenuated by statin therapy.17) Diabetic patients have atherogenic dyslipidemia and hyperglycemia and the potential to generate advanced glycation endproducts.18) Statin therapy could induce the regression of coronary atherosclerosis and there was a strong linear relationship between achieved low density lipoprotein-cholesterol (LDL-C) levels and the course of atherosclerosis.19)20)21)22) So, optimal medical therapy is needed to treat patients with angiographically intermediate lesions with IVUS MLA <4 mm2 with 50-70% of plaque burden. In the present study, TLR at 2-year follow-up tended to be greater in the medical therapy group than in the ZES group if MACE was compared between both groups. However, the trend of difference in TLR would be smaller if we use an optimal medical therapy including high-dose statin. Intensive statin therapy is essential to regress and to stabilize plaque and to prevent the occurrence of MACE at long-term follow-up especially in patients with acute myocardial infarction and diabetes mellitus.
There are several study limitations to be mentioned. First, the sample size in this multicenter study was too small to compare the long-term clinical outcomes of medical therapy and second generation drug-eluting stents in patients with angiographically intermediate lesions. Second, lesion selection and IVUS imaging were performed at the discretion of the individual operators, leading to potential selection biases. Third, patients were not consecutive and only those patients who agreed to participate were enrolled in the present study. Fourth, we did not evaluate the impacts of plaque characteristics on clinical outcomes and we did not evaluate the functional severity of stenosis. Fifth, MACE occurred in only 12 patients during the two years of follow-up, thus the study may be underpowered to identify independent predictors of MACE.
In conclusion, MACE occurred in 9.1% in medical therapy group and in 7.5% in ZES group during two years of follow-up and that was not statistically different between medical therapy and ZES group. A target-vessel related non-fatal myocardial infarction was not developed in both groups and a stent thrombosis did not occur in ZES group. Myocardial infarction, diabetes mellitus and non-statin therapy were associated with the occurrence of two-years MACE in patients with angiographically intermediate lesions with IVUS MLA <4 mm2 with 50-70% of plaque burden. Medical therapy as well as ZES is a good option for the treatment of intermediate lesions. An IVUS evaluation of the non-culprit intermediate lesions without use of pressure wire would be useful to predict a future cardiac event in patients with multi-vessel disease after the percutaneous coronary intervention to the culprit lesion is finished.
Figures and Tables
Fig. 1
Flow diagram of the patients enrolled in MINIATURE study. CAG: coronary angiography, DS: diameter stenosis, IVUS: intravascular ultrasound, MLA: minimum lumen area, ZES: zotarolimus-eluting stent, MACE: major adverse cardiac event.

Fig. 2
Coronary angiography (A) and intravascular ultrasound (B) images in distal circumflex artery in a 55-year-old female who presented with unstable angina. EEM: external elastic membrane, CSA: cross-sectional area.

Table 1
Baseline characteristics, coronary angiography and intravascular ultrasound findings
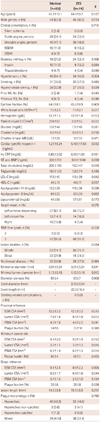
Data are n (%) or mean±SD. ZES: zotarolimus-eluting stent, NSTEMI: non-ST segment elevation myocardial infarction, STEMI: ST-segment elevation myocardial infarction, PCI: percutaneous coronary intervention, hs-CRP: high-sensitivity C-reactive protein, NT-pro-BNP: N-terminal pro-B type natriuretic peptide, LDL-C: low density lipoprotein-cholesterol, HDL-C: high density lipoprotein-cholesterol, TIMI: Thrombolysis in Myocardial Infarction, EEM: external elastic membrane, CSA: cross-sectional area, P&M: plaque plus media
Table 2
Medications and major adverse cardiovascular events at one year and two years of clinical follow-up

Table 3
Independent predictors of plaque progression at minimum lumen area site

Acknowledgments
This study was supported by a grant of the Korean Society of Interventional Cardiology, and a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI13C0163), and a grant of The Korean Society of Cardiology, The Korea Centers for Disease Control and Prevention (2013-E63005-00), and The Korean Health Technology R&D Project (HI13C1527), Ministry of Health & Welfare, and the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MEST) (2012M3A9C6049744), and the National Research Foundation of Korea Grant funded by the Korean Government (2011-0008875), and the Korea Healthcare technology R&D Project, Ministry for Health, Welfare and Family Affairs (HI12C0275), and Chonnam National University Hospital Research Institute of Clinical Medicine (CRI 11080-21), Republic of Korea.
References
1. Briguori C, Anzuini A, Airoldi F, et al. Intravascular ultrasound criteria for the assessment of the functional significance of intermediate coronary artery stenoses and comparison with fractional flow reserve. Am J Cardiol. 2001; 87:136–141.
2. Tobis J, Azarbal B, Slavin L. Assessment of intermediate severity coronary lesions in the catheterization laboratory. J Am Coll Cardiol. 2007; 49:839–848.
3. Abizaid A, Mintz GS, Pichard AD, et al. Clinical, intravascular ultrasound, and quantitative angiographic determinants of the coronary flow reserve before and after percutaneous transluminal coronary angioplasty. Am J Cardiol. 1998; 82:423–428.
4. Pijls NH, Van Gelder B, Van der Voort P, et al. Fractional flow reserve. A useful index to evaluate the influence of an epicardial coronary stenosis on myocardial blood flow. Circulation. 1995; 92:3183–3193.
5. Pijls NH, De Bruyne B, Peels K, et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med. 1996; 334:1703–1708.
6. Baumgart D, Haude M, Goerge G, et al. Improved assessment of coronary stenosis severity using the relative flow velocity reserve. Circulation. 1998; 98:40–46.
7. Ahn JM, Kang SJ, Mintz GS, et al. Validation of minimal luminal area measured by intravascular ultrasound for assessment of functionally significant coronary stenosis comparison with myocardial perfusion imaging. JACC Cardiovasc Interv. 2011; 4:665–671.
8. Koo BK, Yang HM, Doh JH, et al. Optimal intravascular ultrasound criteria and their accuracy for defining the functional significance of intermediate coronary stenoses of different locations. JACC Cardiovasc Interv. 2011; 4:803–811.
9. Kang SJ, Lee JY, Ahn JM, et al. Intravascular ultrasound-derived predictors for fractional flow reserve in intermediate left main disease. JACC Cardiovasc Interv. 2011; 4:1168–1174.
10. Dhawan J, Bray CL. Are Asian coronary arteries smaller than Caucasian? A study on angiographic coronary artery size estimation during life. Int J Cardiol. 1995; 49:267–269.
11. Lip GY, Rathore VS, Katira R, Watson RD, Singh SP. Do Indo-Asians have smaller coronary arteries? Postgrad Med J. 1999; 75:463–466.
12. Effects of tissue plasminogen activator and a comparison of early invasive and conservative strategies in unstable angina and non-Q-wave myocardial infarction. Results of the TIMI IIIB Trial. Thrombolysis in Myocardial Ischemia. Circulation. 1994; 89:1545–1556.
13. Mintz GS, Nissen SE, Anderson WD, et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001; 37:1478–1492.
14. Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011; 364:226–235.
15. Nicholls SJ, Tuzcu EM, Kalidindi S, et al. Effect of diabetes on progression of coronary atherosclerosis and arterial remodeling: a pooled analysis of 5 intravascular ultrasound trials. J Am Coll Cardiol. 2008; 52:255–262.
16. Boyle PJ. Diabetes mellitus and macrovascular disease: mechanisms and mediators. Am J Med. 2007; 120:9 Suppl 2. S12–S17.
17. Takayama T, Hiro T, Ueda Y, et al. Plaque stabilization by intensive LDL-cholesterol lowering therapy with atorvastatin is delayed in type 2 diabetic patients with coronary artery disease-Serial angioscopic and intravascular ultrasound analysis. J Cardiol. 2013; 61:381–386.
18. Stancoven A, McGuire DK. Preventing macrovascular complications in type 2 diabetes mellitus: glucose control and beyond. Am J Cardiol. 2007; 99(11A):5H–11H.
19. Nissen SE, Tuzcu EM, Schoenhagen P, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med. 2005; 352:29–38.
20. Nissen SE, Nicholls SJ, Sipahi I, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA. 2006; 295:1556–1565.
21. Nicholls SJ, Tuzcu EM, Sipahi I, et al. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. JAMA. 2007; 297:499–508.
22. Hong YJ, Jeong MH, Ahn Y, et al. Effect of pitavastatin treatment on changes of plaque volume and composition according to the reduction of high-sensitivity C-reactive protein levels. J Cardiol. 2012; 60:277–282.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download