Abstract
Background and Objectives
Cardiac troponins are associated with increased mortality, even among patients with no coronary artery disease. Elevated cardiac troponin levels are frequently observed in patients without significant coronary lesions, although the mechanism underlying this finding is unclear. The aim of our study was to evaluate the association between the levels of cardiac troponin and coronary flow reserve (CFR).
Subjects and Methods
We evaluated serum cardiac troponin-I in 19 patients (9 female; age 61.9±10.9 year-old). All patients had an ejection fraction >40% and angiographically normal coronary arteries. Simultaneous measurements of fractional flow reserve (FFR), the index of microcirculatory resistance (IMR), and CFR measurements using an intracoronary temperature- and pressure-sensing guidewire under basal conditions and during maximal hyperemia were performed in three vessels: the left anterior descending artery (LAD), left circumflex artery (LCX) and right coronary artery (RCA).
Results
All patients were followed for a median of 13 months. FFR, IMR, and CFR measurements were performed successfully in all subjects. Mean CFRs of LAD, LCX, and RCA were 1.98±1.20, 2.75±2.11, and 4.44±2.51, respectively. Mean IMRs of LAD, LCX and RCA were 33.28±18.78, 29.11±26.70, and 30.55±23.65, respectively. There was a poor correlation between CFR and troponin-I values in each vessel. In selecting the lowest value of CFR in each patient as the corresponding value, the lowest CFR was not associated with troponin-I levels (r=-0.219, p=0.367).
Ischemic heart disease is complex and often involves changes in the epicardial coronary arteries and myocardial microvasculature. Cardiac troponin is a useful tool that may help the clinician in diagnosing myocardial infarction.1) Recent studies have shown that cardiac troponins can detect myocardial injury precisely and may predict short- and long-term mortality, even among those with no significant obstructive coronary artery disease, because high cardiac troponin levels have been associated with anatomical and functional conditions that place patients at risk of future cardiovascular events.2)3)4)
Functional impairment in the coronary microcirculation is thought to be a major pathway in the development of myocardial injury in normal epicardial vessels.5)6) Coronary flow reserve (CFR) provides information on both epicardial and microvascular resistance.7) In the absence of epicardial artery disease, CFR is an important index of coronary microcirculatory function. Recently, coronary thermodilution-derived CFR has been developed, which permits the simultaneous assessment of CFR and fractional flow reserve (FFR) using a single coronary pressure wire.8)9)
Elevation of cardiac troponin is frequently observed in patients without significant coronary lesions, although the mechanism underlying this finding remains unclear.10) The aim of our study was to evaluate the association between the levels of cardiac troponin and CFR.
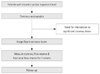
We studied 19 patients with acute chest pain and a lone elevated troponin-I level. None of the patients had significant coronary stenoses (>30% diameter stenosis). Patients were excluded if they had a history of myocardial infarction in the epicardial vessel in the previous 12 months, a Thrombolysis in Myocardial Infarction (TIMI) flow of less than grade 3, severe renal impairment (estimated Glomerular Filtration Rate <30 mL/min), acute inflammatory illness, chronic atrial fibrillation, left ventricular (LV) ejection fraction <40%, previous coronary artery bypass surgery, or significant valvular heart disease. Echocardiography was used to rule out concomitant hypertrophic or dilated cardiomyopathy in all patients. Fig. 1 shows the study design.
The study protocol was approved by the institutional review board of our hospital. Written informed consent was obtained from all patients.
Troponin-I was measured using an Abbott AxSYM analyzer (Abbott Laboratories, Abbott Park, IL, USA) by mass immunoassay with a normal upper limit of 0.3 ng/mL, as specified by the manufacturer. According to the manufacturer's recommendations and based on our analyses, a definite elevation of troponin-I was defined as a troponin-I level higher than 0.3 ng/mL.11) The 99th percentile of the URL was calculated to be 0.3 ng/mL, based on a sample of 100 healthy patients from the hospital's catchment area. Detectable elevation was defined as between 0.10 and 0.30 ng/mL.11) Study participants were divided into two groups, based on the troponin-I level.
Coronary angiography was performed with a femoral artery approach. At the beginning of the procedure, heparin (5000 units) was routinely administered intra-arterially. Coronary lesions were assessed by visual estimation as well as with quantitative coronary angiography. The provocation test was conducted with ergonovine in all patients after identifying insignificant lesions during the angiogram.
After the injection of intracoronary nitroglycerin (200 µg), simultaneous measurements of FFR and CFR were performed for all arteries. A 6 Fr or 7 Fr coronary guiding catheter without side holes was used to engage the selected coronary artery. A 5 Fr sheath was placed in the right femoral vein for the injection of adenosine. A 0.014 coronary temperature and pressure-sensing guidewire (PressureWire Certus, St. Jude Medical, MN, USA) was calibrated for the pressure recording, and then equalized with the aortic pressure in the guiding catheter. Next, the wire was advanced to the distal third part of the artery. For the induction of maximal hyperemia, intravenous adenosine was administered via the right femoral vein (140 mg/kg/min).
Fractional flow reserve, index of microcirculatory resistance (IMR), and CFR were measured under maximal hyperemia, induced by adenosine. FFR was calculated as the mean distal coronary pressure divided by the mean aortic pressure during hyperemia. For the measurement of CFR, basal coronary flow under basal conditions was determined by intracoronary administration of 3 mL of room-temperature saline, three times in succession manually (3 mL/s). Then, maximal hyperemia was induced, and three additional room temperature saline boluses of 3 mL were administered intracoronarily for the determination of peak coronary flow, presented as peak mean transit time. During saline injection, careful attention was given to the position of the guiding catheter and the distal sensor. CFR was calculated automatically from the ratio of the mean transit times during hyperemia and baseline. Adenosine was turned off for 5 minutes after physiological measurements for each artery and the following vessels were measured in sequence: left anterior descending artery (LAD), left circumflex artery (LCX), and right coronary artery (RCA). After the evaluation of FFR and CFR, IMR was calculated. In all vessels, a simplified method of calculating IMR was used, as follows:
where Pd and Tmn are the mean hyperemic distal coronary pressure and hyperemic transit time, respectively.
Data are expressed as means±standard deviations for continuous variables and as numbers (%) for categorical variables, as appropriate. Comparisons of mean values were performed using Student's or the paired t-test. For comparisons of discrete variables, the χ2 test was used. Correlations between CFR and FFR variables were analyzed using a Spearman correlation analysis. CFR was compared with troponin-I by linear regression analysis. A p<0.05 was considered to indicate statistical significance.
In total, 19 patients with TIMI 3 flow at baseline angiography were enrolled. The baseline characteristics of all patients are presented in Table 1. The mean age was 61.9±10.9 years, and nine (47.4%) of the patients were women. None of the patients had previous coronary revascularization, such as stenting or coronary bypass surgery. The overall population was classified into definite and detectable elevation according to troponin-I level. Clinical characteristics of two groups, based on the troponin-I concentration, are shown in Table 2. Of the 19 patients, 15 had a definite elevation in troponin-I, with a median level of 2.34 ng/mL.
The study was completed successfully according to the protocol in all populations. In total, 52 arteries were studied in 19 patients without evidence of significant coronary artery lumen obstruction. In all study arteries, FFR values were above 0.8.
Coronary flow reserve and IMR were available from 50 arteries (87.7% of all vessels). The main reasons for not obtaining adequate data were chest discomfort after adenosine infusion and small vessel size. The CFR of LAD, LCX, and RCA were 1.98±1.20, 2.75±2.11 and 4.44±2.51, respectively (Table 1). There was a significant difference in CFR between LAD and RCA (p=0.003). However, no significant difference was observed between the three vessels in IMR measurements. Coronary physiological data are outlined in Table 2.
There was no difference with regard to CFR, FFR, or IMR between the two groups (Table 2). Fig. 2 shows a poor correlation between CFR and troponin-I value in each vessel. Even in selecting the lowest value of CFR in each patient as the corresponding value, the lowest CFR was not associated with the troponin-I level (Fig. 3).
Additionally, the correlation between IMR and troponin-I was not significant in LAD (r=0.378, p=0.111), LCX (r=0.468, p=0.068), or RCA (r=0.044, p=0.881) (Fig. 4). However, in selecting the highest value of IMR in each patient as the corresponding value, the highest IMR was significantly higher in patients with definite elevation compared with detectable elevation (Table 2). Among physiological variables, only the highest IMR correlated with troponin-I concentration (Fig. 5).
The main finding of our investigation is that the CFR, using a thermodilution technique, is poorly associated with troponin-I level in patients with troponin-I elevation, but having no significant epicardial coronary artery stenosis. In published articles, cardiac biomarker measurement has been demonstrated be a statistically significant independent predictor of intermediate and long-term outcomes for cardiac events.2)3)4) Furthermore, minor elevations in troponin levels below the 99th percentile of the URL have been associated with mortality and adverse cardiac events.11) Thus, understanding their mechanism will be important in patients with normal coronary arteries. Different pathogenic mechanism are responsible for troponin-I elevation in patients with insignificant coronary lesions. Impaired coronary microcirculation3)12) is one that can contribute to myocardial ischemia in patients with angiographically normal coronary arteries, and can be aggravated by microcirculatory stagnation, direct impairment, vasoconstriction, and thrombosis.13)
Coronary flow reserve is a commonly used index for coronary microvascular function. The concept of CFR, introduced 50 years ago by Coffman and Gregg,7) provides a method for describing the capacity of the coronary circulation to conduct maximal hyperemic blood flow. Previous studies have shown that the presence of an abnormal CFR is related to a worse outcome in various disorders,14)15) and CFR provides significant data on the improvement in myocardial function in patients with previous myocardial infarction.16)17) It has also been demonstrated that the severity of impairment is correlated with cardiac markers.18) Thus, CFR has been considered to be a marker of coronary microvascular response. However the studies have some limitations. First, CFR assessment was performed only in the LAD, leading to caution in interpreting the data for general microvascular function, although it did not seem to have any influence.14)15)18) Second, CFR was measured by transthoracic echocardiography, in which the accuracy to detect coronary flow is influenced by image quality and angle dependence of Doppler velocities.14)15)17)
We tried to measure CFR in three vessels in each patient, because the value of LAD CFR may not be completely appropriate for identifying a reduced CFR response. Also, an invasive method was used for the assessment of CFR to overcome non-invasive methodological limitations. However, our trial showed that CFR did not correlate with troponin-I concentrations. This finding differs from studies of stable patients with minor or normal LV function.19) This result may be due to hemodynamic dependence of CFR and submaximal hyperemia with inadequate adenosine infusion.20)21) CFR is influenced by hemodynamics and the coronary microcirculation.20) Hyperemic flow, which is linearly related to coronary perfusion pressure, depends on total coronary resistance, whereas baseline flow is influenced by several factors, including myocardial oxygen demand and vasomotor tone. Additionally, the accuracy of CFR can be limited in a submaximal hyperemic condition.21)
The present study also showed that the CFR value was significantly lower in the LAD than in the RCA. Because CFR is a summed response of both the epicardial and microvascular flows, a normal CFR indicates that both the epicardial and minimally achievable microvascular bed resistances are low and normal.21) When abnormal, CFR does not indicate which component is affected. Thus, CFR is the best available tool to assess the microcirculation in the absence of epicardial coronary narrowings. In our study, the mean FFR in LAD was 0.89, lower than the results of LCX and RCA. These results may suggest that most of the subjects likely had worse coronary atherosclerosis in the LAD. This finding also may be due to submaximal hyperemia with inadequate adenosine infusion, because CFR measurements in each patient were attempted first in the LAD.
Recently, IMR was reported to be more useful in evaluating the microcirculation than CFR.22)23)24) The present study demonstrated that the highest IMR in each patient correlated with the troponin-I level. This may suggest that the coronary microcirculation is impaired in patients with troponin-I elevation, and IMR can be a territory-specific physiological parameter. IMR seems to be helpful in the functional assessment of coronary microvasculature.
This study has several limitations. The first major limitations were possible selection bias and the small sample size without objective references. Second, there was no control group with which to compare the CFR value of negative troponin-I patients. Invasive physiological measurements were only performed in patients with troponin-I elevation. Third, as the mean age of our patients was 62, most of them likely had some degree of coronary atherosclerosis. Even though we excluded patients with significant coronary artery disease, the influence of an atherosclerotic component in the results could not be excluded. Fourth, clinical diagnoses in the population were heterogeneous. Finally, our study could not establish the clinical relevance of CFR due to the small sample size.
In conclusion, the correlation between CFR, using a thermodilution technique, and troponin-I levels was not significant in patients with no significant coronary artery disease. CFR may be limited to estimating the microcirculation. Further studies of larger populations with longer-term follow-up are needed to more fully understand microvascular dysfunction.
Figures and Tables
Fig. 2
Scatterplot of troponin-I versus CFR in LAD (A), LCX (B), and RCA (C). CFR: coronary flow reserve, LAD: left anterior descending artery, LCX: left circumflex artery, RCA: right coronary artery.

Fig. 3
Correlation between the lowest CFR value measured by thermodilution and cardiac troponin-I (CFR: horizontal axis; cardiac troponin-I: vertical axis). CFR: coronary flow reserve.

Fig. 4
Scatterplot of troponin-I versus IMR in LAD (A), LCX (B), and RCA (C). IMR: index of microcirculatory resistance, LAD: left anterior descending artery, LCX: left circumflex artery, RCA: right coronary artery.

Fig. 5
Correlation between the highest IMR value measured by thermodilution and cardiac troponin-I (IMR: horizontal axis; cardiac troponin-I: vertical axis). IMR: index of microcirculatory resistance.

Table 1
Baseline characteristics of study population
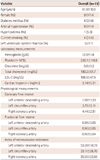
Table 2
Clinical and physiological data in patients with definite and detectable elevation of cardiac troponin-I

Acknowledgments
This study was supported by a grant from the Korean Society of Cardiology (2011), Republic of Korea.
References
1. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012; 60:1581–1598.
2. Leonardi S, Thomas L, Neely ML, et al. Comparison of the prognosis of spontaneous and percutaneous coronary intervention-related myocardial infarction. J Am Coll Cardiol. 2012; 60:2296–2304.
3. Agewall S, Giannitsis E, Jernberg T, Katus H. Troponin elevation in coronary vs. non-coronary disease. Eur Heart J. 2011; 32:404–411.
4. Eggers KM, Lagerqvist B, Venge P, Wallentin L, Lindahl B. Persistent cardiac troponin I elevation in stabilized patients after an episode of acute coronary syndrome predicts long-term mortality. Circulation. 2007; 116:1907–1914.
5. Leung DY, Leung M. Non-invasive/invasive imaging: significance and assessment of coronary microvascular dysfunction. Heart. 2011; 97:587–595.
6. Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007; 356:830–840.
7. Coffman JD, Gregg DE. Reactive hyperemia characteristics of the myocardium. Am J Physiol. 1960; 199:1143–1149.
8. De Bruyne B, Pijls NH, Smith L, Wievegg M, Heyndrickx GR. Coronary thermodilution to assess flow reserve: experimental validation. Circulation. 2001; 104:2003–2006.
9. Barbato E, Aarnoudse W, Aengevaeren WR, et al. Validation of coronary flow reserve measurements by thermodilution in clinical practice. Eur Heart J. 2004; 25:219–223.
10. Eggers KM, Lind L, Ahlström H, et al. Prevalence and pathophysiological mechanisms of elevated cardiac troponin I levels in a population-based sample of elderly subjects. Eur Heart J. 2008; 29:2252–2258.
11. van den Bos EJ, Constantinescu AA, van Domburg RT, Akin S, Jordaens LJ, Kofflard MJ. Minor elevations in troponin I are associated with mortality and adverse cardiac events in patients with atrial fibrillation. Eur Heart J. 2011; 32:611–617.
12. Layland JJ, Whitbourn RJ, Burns AT, et al. The index of microvascular resistance identifies patients with periprocedural myocardial infarction in elective percutaneous coronary intervention. Heart. 2012; 98:1492–1497.
13. Merx MW, Weber C. Sepsis and the heart. Circulation. 2007; 116:793–802.
14. Nakanishi K, Fukuda S, Shimada K, et al. Prognostic value of coronary flow reserve on long-term cardiovascular outcomes in patients with chronic kidney disease. Am J Cardiol. 2013; 112:928–932.
15. Cortigiani L, Rigo F, Gherardi S, et al. Coronary flow reserve during dipyridamole stress echocardiography predicts mortality. JACC Cardiovasc Imaging. 2012; 5:1079–1085.
16. Beleslin B, Ostojic M, Djordjevic-Dikic A, et al. The value of fractional and coronary flow reserve in predicting myocardial recovery in patients with previous myocardial infarction. Eur Heart J. 2008; 29:2617–2624.
17. Løgstrup BB, Høfsten DE, Christophersen TB, et al. Association between coronary flow reserve, left ventricular systolic function, and myocardial viability in acute myocardial infarction. Eur J Echocardiogr. 2010; 11:665–670.
18. Takashio S, Yamamuro M, Izumiya Y, et al. Coronary microvascular dysfunction and diastolic load correlate with cardiac troponin T release measured by a highly sensitive assay in patients with nonischemic heart failure. J Am Coll Cardiol. 2013; 62:632–640.
19. Sicari R, Rigo F, Cortigiani L, Gherardi S, Galderisi M, Picano E. Additive prognostic value of coronary flow reserve in patients with chest pain syndrome and normal or near-normal coronary arteries. Am J Cardiol. 2009; 103:626–631.
20. Ng MK, Yeung AC, Fearon WF. Invasive assessment of the coronary microcirculation: superior reproducibility and less hemodynamic dependence of index of microcirculatory resistance compared with coronary flow reserve. Circulation. 2006; 113:2054–2061.
21. Kern MJ, Lerman A, Bech JW, et al. Physiological assessment of coronary artery disease in the cardiac catheterization laboratory: a scientific statement from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology. Circulation. 2006; 114:1321–1341.
22. Lim HS, Yoon MH, Tahk SJ, et al. Usefulness of the index of microcirculatory resistance for invasively assessing myocardial viability immediately after primary angioplasty for anterior myocardial infarction. Eur Heart J. 2009; 30:2854–2860.
23. Oh JH, Kim C, Ahn J, et al. The relationship between microcirculatory resistance and fractional flow reserve in patients with acute Myocardial infarction. Korean Circ J. 2013; 43:534–540.
24. Fearon WF, Shah M, Ng M, et al. Predictive value of the index of microcirculatory resistance in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2008; 51:560–565.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download