Abstract
Pacemaker lead endocarditis is treated with total removal of the infected device and proper antibiotics. The outcomes of patients undergoing percutaneous lead extraction for large vegetations (>2 cm) have not yet been shown. In this case report, we present two patients with pacemaker lead endocarditis with large vegetations of maximum diameter 2.4 cm and 3.2 cm. The first patient had multiple vegetations attached to the tricuspid and mitral valves and developed septic emboli to the brain, lung, and liver. The second patient had a large, persistent vegetation on the tricuspid valve, even two weeks after complete removal of the leads. Both patients were successfully treated with transvenous pacemaker lead removal and antibiotics.
Currently, more than 3 million implantable cardiac pacing systems exist worldwide.1)2) The estimated rate of infection after permanent endocardial lead implantation is between 1% and 2%.3) If treatment of pacemaker lead infection is delayed, complications such as destruction of the tricuspid valve, septic pulmonary embolism, and consecutive abscess-forming pneumonia can occur.4) When septic emboli are dislodged or when lead infection is complicated by large vegetations, the ideal therapeutic strategy (either transvenous removal or surgical ablation) is not clear but likely depends on the size of the vegetation. The outcomes of patients undergoing transvenous lead removal in endocarditis with large vegetation have not yet been elucidated.4)
We present two patients with pacemaker lead endocarditis with large vegetations (>2 cm). Both cases were successfully managed with transvenous lead removal.
A 63-year-old male was admitted for fever and confusion of three days. He had undergone pacemaker (VDD type) implantation for symptomatic sick sinus syndrome three months prior.
Upon admission, the patient was febrile with a temperature of 38.3℃, confused and had dysarthric speech. Physical examination was remarkable for warmth, erythema, and tenderness of the skin overlying the pacemaker pocket, which was exposed. His white blood cell (WBC) count was 18.40×103/µL, and C-reactive protein (CRP) concentration was 148.27 mg/L. Transthoracic echocardiogram (TTE) revealed vegetations attached to the tricuspid valve (2.4×1.1 cm) (Fig. 1A, arrow), right atrium (0.8×0.8 cm) (Fig. 1A, broken arrow), and mitral valve (0.7×0.5 cm) (Fig. 1B, arrow). Brain magnetic resonance imaging results were consistent with multiple acute embolic infarcts (Fig. 2A). Computed tomography scan of the chest and abdomen showed multiple cavitary nodules in both lungs (Fig. 2B) and small, low-attenuated lesions in segment 6 of the liver (Fig. 2C), suggesting septic embolism.
On the same day, the patient underwent transvenous removal of the pacemaker lead (Fig. 1E and F). Pocket site swab cultures and three separate sets of blood cultures were collected and later revealed methicillin-resistant, Staphylococcus aureus. The patient was treated with vancomycin 1000 mg every 12 hours. On the tenth day, blood cultures were negative for the first time. The patient's fever relapsed at day 14 and three of three blood cultures grew Candida tropicalis (C. tropicalis). Repeated blood cultures were positive for C. tropicalis until day 18. Antifungal treatment was started intravenously and continued for a total of six weeks, including four weeks with liposomal amphotericin B 350 mg/day (5 mg/kg) and two weeks with fluconazole 400 mg/day. After 22 days, vegetations of tricuspid valve and right atrium were no longer visible on TTE (Fig. 1C and D). The patient was discharged after eight weeks of admission and no relapse was documented at the six months clinical visit.
A 19-year-old woman was admitted for chills of three days. She had a history of pacemaker (VDD type) implantation five years prior for high-degree atrioventricular block with recurrent syncope.
Upon admission she was afebrile and did not reveal pacemaker skin redness or tenderness. Her WBC count was 12.75×103/µL and CRP concentration was 26.4 mg/L. TTE revealed a large vegetation (2.5×1.7 cm) attached to the tricuspid valve (Fig. 3A). We began treatment with intravenous vancomycin 1000 mg every 12 hours.
On the third day, three different sets of blood cultures grew Staphylococcus epidermidis and blood cultures were positive until day 12. At day 15, the size of the largest vegetation increased to 3.2×0.8 cm. We then completely removed the previous pacemaker and leads (Fig. 3B) transvenously with a temporary pacemaker. Blood cultures were negative for the first time at day 17, but TTE revealed no interval change in vegetation size until day 32. Despite the lack of interval vegetation size change, a new pacemaker (VDD type) was implanted at day 32 (Fig. 3C). After successful re-implantation, treatment with intravenous vancomycin was continued for a total of six weeks and TTE showed complete resolution of the vegetation. At the five-year follow-up, the TTE showed no relapse (Fig. 3D).
Pacemaker lead endocarditis is considered to be the most severe form of endocarditis.5) In addition to proper antimicrobial therapy, complete removal of the device is required and antimicrobial therapy alone is often unsuccessful and associated with a high mortality rate.6) A variety of percutaneous lead-removal techniques are available, and only a small minority of patients require open cardiovascular surgery for complete device removal.7) Although there has been no study comparing transvenous and surgical removal of leads in large vegetation pacemaker endocarditis, removal via open thoracotomy has been advocated for leads with large vegetations due to the possibility of dislodging emboli. Previous literature has analyzed the perceived risk of embolic events in the presence of large vegetations (>1.0 cm), a relative contraindication to transvenous removal.7)8) Surgical removal of the device has been suggested in these circumstances to avoid complications such as pulmonary embolism.7) Ruttmann et al.4) on the other hand, reported that the transvenous extraction of endocardial leads with large vegetations (>1.0 cm) is feasible. They described that although pulmonary embolism does occur, it does not influence the survival or length of hospitalization. Consistent with this report, in our two cases with large vegetations (maximum diameter of 3.2 cm), percutaneous lead extraction was performed successfully without complications such as tears of the tricuspid valve, hemothorax, tamponade, pulmonary embolism, or lead migration. Our cases also show that percutaneous removal of the infected leads is effective even in patients with multi-organ septic emboli.
Our first patient no longer exhibited the signs and symptoms of sick sinus syndrome after complete removal of the device, so we did not implant a new pacemaker. The second patient needed a new pacemaker; however, the vegetations were persistently observed even two weeks after complete removal of the leads. As there were no signs of infection, we re-implanted the new device.
Our report suggests that, although pacemaker lead endocarditis may be accompanied by large vegetations and lung and brain emboli, it can be treated successfully with transvenous lead extraction and proper antibiotics.
Figures and Tables
Fig. 1
TTE demonstrating a 2.4×1.1 cm vegetation attached to the TV posterior leaflet (arrow) and a 0.8×0.8 cm vegetation attached to the RA-free wall (broken arrow) (A). A 0.7×0.5 cm vegetation attached to the MV anterior mitral leaflet (B). Disappearance of vegetations on both TV posterior leaflet and RA-free wall (C). Disappearance of MV anterior leaflet vegetation (D). Chest radiograph before device removal (E) and after device removal (F). TTE: transthoracic echocardiography, LA: left atrium, LV: left ventricle, RA: right atrium, TV: tricuspid valve, MV: mitral valve.
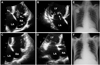
Fig. 2
A: brain MRI showing multiple acute infarcts in the bilateral cerebral hemispheres, suggestive of embolism. B: a CT scan of the chest showing small and solid cavitary nodules, suggesting septic embolism. C: a CT scan of the abdomen showing small, ill-defined, low-attenuated lesions in segment 6 of the liver, which can be inflammatory lesions but is difficult to characterize with single-phase CT. CT: computed tomography, MRI: magnetic resonance imaging.
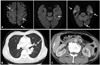
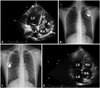
Fig. 3
A: TTE on admission demonstrating a 2.5×1.7 cm TV anterior leaflet vegetation (broken arrow) and a shaggy shaped 1.7×0.8 cm mass attached to the right atrium lateral wall (arrow), concerning for vegetation. Chest radiograph, before infected pacemaker removal (B) and after new pacemaker implantation (C). Follow-up TTE five years later showing disappearance of vegetations (D). TTE: transthoracic echocardiography, LA: left atrium, LV: left ventricle, RA: right atrium, TV: tricuspid valve.
Acknowledgments
This study was supported in part by research grants from the Korean Heart Rhythm Society (2011-3), the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2012-0007604, 2012-045367), and a grant of the Korean Healthcare Technology R&D Project funded by the Ministry of Health & Welfare (A121668).
References
1. Chua JD, Wilkoff BL, Lee I, Juratli N, Longworth DL, Gordon SM. Diagnosis and management of infections involving implantable electrophysiologic cardiac devices. Ann Intern Med. 2000; 133:604–608.
2. Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009--a World Society of Arrhythmia's project. Pacing Clin Electrophysiol. 2011; 34:1013–1027.
3. Klug D, Balde M, Pavin D, et al. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation. 2007; 116:1349–1355.
4. Ruttmann E, Hangler HB, Kilo J, et al. Transvenous pacemaker lead removal is safe and effective even in large vegetations: an analysis of 53 cases of pacemaker lead endocarditis. Pacing Clin Electrophysiol. 2006; 29:231–236.
5. Sohail MR, Uslan DZ, Khan AH, et al. Management and outcome of permanent pacemaker and implantable cardioverter-defibrillator infections. J Am Coll Cardiol. 2007; 49:1851–1859.
6. Camus C, Leport C, Raffi F, et al. Sustained bacteremia in 26 patients with a permanent endocardial pacemaker: assessment of wire removal. Clin Infect Dis. 1993; 17:46–55.
7. Meier-Ewert HK, Gray ME, John RM. Endocardial pacemaker or defibrillator leads with infected vegetations: a single-center experience and consequences of transvenous extraction. Am Heart J. 2003; 146:339–344.
8. Klug D, Lacroix D, Savoye C, et al. Systemic infection related to endocarditis on pacemaker leads: clinical presentation and management. Circulation. 1997; 95:2098–2107.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download