Abstract
Congenital pericardial defects are rare and asymptomatic for both partial and complete defects. However, some patients can experience syncope, arrhythmia, and chest pain. When a patient experiences a symptom, it may be caused by herniation and dynamic compression or torsion of a heart structure including the coronary arteries. Diagnosis of a congenital pericardial defect may be difficult, especially in old patients with concomitant coronary artery disease. The clinical importance of congenital pericardial defect has not been stressed and congenital pericardial defects are regarded as benign, but in this case, pericardial defect was responsible for myocardial ischemia. The authors report a case of partial congenital pericardial defect causing herniation and dynamic compression of the coronary arteries, presenting as an acute coronary syndrome in an old man, with an emphasis on the unique features of the coronary angiogram that support the diagnosis of partial pericardial defects.
Congenital pericardial defects are rare. They are observed in roughly 1 in 14000 patients who undergo thoracotomies and necropsies.1) Patients with congenital pericardial defects are either asymptomatic or occasionally present with varied symptoms, chest pain being the most prevalent symptom.2) The chest pain may be caused by compromised blood flow by herniation and dynamic compression or torsion of a heart structure including the coronary arteries. However, the diagnosis of congenital pericardial defect may be difficult or delayed because of various diseases that present with chest pain.
This case report summarizes our experience in the diagnosis and treatment of a patient who had a congenital pericardial defect with concomitant coronary artery disease.
A 61-year-old man visited our hospital with the chief complaints of chest pain on exertion and an intermittent chest pain that was aggravated when he rested in a left decubitus position. He had risk factors for coronary artery disease: dyslipidemia, hypertension, and diabetes. He also had a history of chest trauma, a left rib fracture that occurred in a traffic accident 15 years previously. An initial evaluation of his chest radiograph showed levoposition of his heart and an unusual cardiac contour with a bulging left ventricle (Fig. 1). An electrocardiography showed an incomplete right bundle branch block and a clockwise rotation in the precordial leads. An echocardiography showed an estimated left ventricular ejection fraction of 50-55%, with regional wall motion abnormalities of the basal to mid inferior and inferolateral walls. It also showed a focal external compression of the basal posterolateral wall. His serum troponin-T level was also slightly elevated (0.023 ng/mL). As he had coronary risk factors including elevation of the serum troponin-T level and complained of chest pain on exertion with echocardiographic regional wall motion abnormalities, we performed early elective coronary angiography without noninvasive testing. The angiogram showed two-vessel coronary artery disease involving the left anterior descending artery (LAD) and left circumflex artery (LCX), with a geographically circumferential phasic diastolic compression of three obtuse marginal branches, right ventricular branches, and the posterior descending artery (PDA) (Fig. 2).
At first, we considered stenting the LAD as the significant culprit lesion. However, there was a possibility that phasic diastolic obstruction of the epicardial coronary arteries could have caused the chest pain. This feature was consistent with the echocardiographic findings showing regional wall motion abnormalities of the LCX and right coronary artery territories. In addition, a geographically circumferential phasic diastolic compression of the coronary arteries like myocardial bridging suggested a linear extracardiac constriction like a band strangulating the heart between the basal and the mid-ventricular level. Because he had a previous chest trauma history, there was a possibility that cicatrical changes could have caused extracardiac constriction. We decided against performing percutaneous coronary intervention for the mid-LAD lesion. Therefore, to confirm our assumption, a computed tomography (CT) was done, which showed a left-sided movement of the heart, adherence of the heart to the chest wall, and a partial pericardial defect around the mid-ventricular level with strangulation of the mid-LAD, LCX, and PDA (Fig. 3). There was no specific intracardiac abnormality other than the pericardial defect on cardiac CT. Myocardial single photon emission computerized tomography was also done and it showed a reversible perfusion defect at the mid anterior, entire anterolateral and mid inferolateral walls.
The operative findings revealed a partial congenital pericardial defect just around the middle ventricular level. Both ventricles were herniated to the left pleural cavity, and there was no epicardial adhesion or scar change. There were also multiple coronary arteries strangulated on the epicardial surface of both ventricles caused by the thickened hard circumferential pericardial edge (Fig. 4). The anterior portion of this band-like thickened pericardial edge was excised in order to release the external compression of multiple coronary arteries. The posterior portion of the pericardial edge was left to prevent phrenic nerve injury. An off-pump coronary artery bypass surgery (CABG) of the left internal thoracic artery (free graft to ascending aorta) to the mid-LAD was done concomitantly considering the presence of significant stenosis with heavy calcification of the mid-LAD, which can cause ischemia.
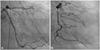
After the CABG with partial pericardiectomy, a follow-up coronary angiogram was performed to evaluate the possibility of remaining strangulation by chronic cicatricial changes of the epicardium beneath the thickened hard circumferential pericardial edge, but there was no finding of previous dynamic obstruction of the coronary arteries (Fig. 5). A follow-up transthoracic echocardiography was also done and the regional wall motion abnormalities and external compression which were observed in the previous study were no longer seen. The patient was discharged without complications, and he is currently symptom free.
Patients with congenital pericardial defects are usually asymptomatic and have a good prognosis. However some patients experience syncope, arrhythmia, and chest pain. The most prevalent symptom is angina-like chest pain that can also appear after postural changes.3)4) The exact mechanism of the chest pain is unclear. Fisher and Ehrenhaft3) suggested possible etiologies as 1) strangulation of the fibrous pericardial rim on the coronary arteries causing myocardial ischemia; 2) torsion or strain of the great vessels; 3) lack of a cushioning effect of the pericardium, allowing the heart to pound freely on the overlying lung or chest; and 4) tension in the pleuropericardial adhesions that form in the absence of the parietal pleuropericardium.5)
The diagnosis of a congenital pericardial defect may be especially difficult in patients with concomitant coronary artery disease like in this case. In a partial pericardial defect case, chest radiography shows a convex prominence of the left cardiac border without electrocardiographic evidence of left ventricular hypertrophy.6)7) The electrocardiography displays an incomplete right bundle branch block pattern with clock wise rotation in left sided congenital pericardial defect cases.6)7) Cardiac CT, magnetic resonance imaging and thoracoscopy are the usual confirmative diagnostic tools.8) Coronary angiography can show dynamic obstruction of the coronary artery during the diastolic phase that is relieved during the systolic phase.
In conclusion, patients with congenital pericardial defects usually have a good prognosis. However, coronary arteries strangulated by the dense fibrous rim of a pericardial defect can produce clinical symptoms, suggesting that this feature was the cause of cardiac ischemia in our case. The clinical diagnosis may be suggested by the characteristic coronary angiographic findings such as the geographically circumferential diastolic compression of the coronary arteries. Most of all, clinicians should strongly suspect this condition when a combination of these symptoms and findings is present to make a confirmative diagnosis of congenital pericardial defect.
Figures and Tables
Fig. 1
Chest radiograph. Chest posteroanterior view shows levoposition of heart with a bulging of the left ventricle.

Fig. 2
Diagnostic coronary angiography. Coronary angiogram shows geographically circumferential phasic diastolic external compression of the obtuse marginal branches of the left circumflex artery (white arrows), right ventricular branches and the posterior descending artery of the right coronary artery (black arrows). A: left anterior oblique-caudal view. B: right anterior oblique-caudal view.
Fig. 3
Computed tomography of the heart. Cardiac CT shows a circumferential groove of the heart caused by external compression and impingement of the coronary arteries (arrows). A: cross sectional view. B: three-dimensional reconstruction view.
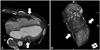
Fig. 4
The operative findings. The operative findings shows a rolled up thickened circumferential pericardial edge and the epicardial groove created by the dense fibrous rim of the pericardium just around the mid-level of the left ventricle.
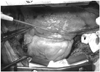
Fig. 5
A follow-up coronary angiography. Coronary angiogram show no features of the previous dynamic occlusion of the coronary arteries which were shown in Fig. 2. A: left anterior oblique-caudal view. B: right anterior oblique-caudal view.
References
1. Rusk RA, Kenny A. Congenital pericardial defect presenting as chest pain. Heart. 1999; 81:327–328.
2. Kojima S, Nakamura T, Sugiyama S, et al. Cardiac displacement with a congenital complete left-sided pericardial defect in a patient with exertional angina pectoris. Angiology. 2004; 55:445–449.
3. Fisher FD, Ehrenhaft JL. Congenital pericardial defect. JAMA. 1964; 188:78–81.
4. Amiri A, Weber C, Schlosser V, Meinertz TH. Coronary artery disease in a patient with a congenital pericardial defect. Thorac Cardiovasc Surg. 1989; 37:379–381.
5. Nguyen DQ, Wilson RF, Bolman RM 3rd, Park SJ. Congenital pericardial defect and concomitant coronary artery disease. Ann Thorac Surg. 2001; 72:1371–1373.
6. Connolly HM, Click RL, Schattenberg TT, Seward JB, Tajik AJ. Congenital absence of the pericardium: echocardiography as a diagnostic tool. J Am Soc Echocardiogr. 1995; 8:87–92.
7. Spodik D. Congenital abnormalities of the pericardium. The Pericardium: A Comprehensive Review. 1st ed. New York: Marcel Dekker;1997. p. 65–75.
8. Spodik D. Pericardial diseases. In : Braunwald E, Zipes DP, Libby P, editors. Heart Disease: A Textbook of Cardiovascular Medicine. 6th ed. Philadelphia: Saunders;2001. p. 1823–1876.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download