Abstract
Background and Objectives
The electrophysiological properties associated with favorable outcome of radiofrequency catheter ablation (RFCA) for idiopathic ventricular arrhythmia (VA) originating from the papillary muscle (PM) remain unclear. The purpose of this study was to investigate the relationships of electrophysiological characteristics and predictors with the outcome of RFCA in patients with VAs originating from PM in the left ventricle (LV).
Subjects and Methods
Twelve (4.2%) of 284 consecutive patients with idiopathic VAs originating from LV PM were assessed. The electrophysiological data were compared between the patients in the successful group and patients in the recurrence group after RFCA.
Results
In 12 patients with PM VAs, non-sustained ventricular tachycardias (VTs, n=6), sustained VTs (n=4) and premature ventricular complexes (n=2) were identified as the presenting arrhythmias. Seven of eight patients showing high-amplitude discrete potentials at the ablation site had a successful outcome (85.7%), while the remaining four patients who showed low-amplitude fractionated potentials at the ablation site experienced VA recurrence. The mean duration from onset to peak downstroke (Δt) on the unipolar electrogram was significantly longer in the successful group than in the recurrence group (58±8 ms vs. 37±9 ms, p=0.04). A slow downstroke >50 ms of the initial Q wave on the unipolar electrogram at ablation sites was also significantly associated with successful outcome (85.7% vs. 25.0%, p=0.03).
The papillary muscle (PM) has been implicated as a potential site of arrhythmogenesis that may contribute to ventricular tachycardia (VT) or fibrillation in animal models1-3) and as an arrhythmogenic focus or part of the reentrant circuit of VT in patients with prior myocardial infarction.4-6) Recently, several studies have reported that idiopathic focal ventricular arrhythmias (VAs) can originate from the anterior or posterior PM in the left ventricle (LV)7-11) or right ventricle.12) Another study reported that the presence of Purkinje potentials at the ablation site was associated with successful outcome of catheter ablation.13) In idiopathic LV fascicular VT (ILVT), the Purkinje network in the vicinity of the PM is also involved in the related mechanism.14-16) In previous studies, radiofrequency catheter ablation (RFCA) of PM VAs was difficult compared to ILVT.8)9) The purpose of this study was to assess the relationships of electrophysiological characteristics and predictors of RFCA outcome in patients with VAs originating from the PM in the LV.
A total of 284 consecutive patients (153 females and 131 males; mean age 44±14 years) with idiopathic VA underwent RFCA at our institution between January 2006 and June 2012. The presenting VAs included sustained monomorphic VT in 81 patients, non-sustained VT {defined as ≥3 consecutive premature ventricular complexes (PVCs) lasting <30 seconds} in 35 patients and frequent PVCs in 168 patients.
The study population consisted of 12 consecutive patients without structural heart disease who underwent catheter ablation of VAs with the ablation site at the PM in the LV. Two patients who had symptomatic frequent PVC (PVC burden ≥10%/day) despite taking antiarrhythmic medications were included. The Local Institutional Review Board approved the study, and written informed consent was obtained from all patients. To evaluate the predictors affecting clinical outcome, the patients were divided into two groups according to result after RFCA: successful and recurrence groups. We defined successful catheter ablation as no recurrence of symptomatic VAs in the absence of any antiarrhythmic drugs, and recurrence was defined when PVC burden >1%/day was identified along with symptoms. PVC burden was defined as the percentage of PVCs relative to the total number of QRS complexes on 24-hour Holter monitoring.
Twelve-lead electrocardiograms (ECGs) of the arrhythmias were analyzed for QRS morphology, QRS width, axis, precordial transition and the presence of a notch in V 1 to V 6. Notches were defined as deflections in the QRS complex other than a triphasic pattern. We defined the presence of notches when they were clearly demonstrated in three or more consecutive precordial leads.
All patients underwent an electrophysiological study and RFCA. Prior to the procedure, all antiarrhythmic drugs were discontinued for more than five half-lives. An electrophysiological study was performed using two quadripolar catheters placed in the His bundle region and the right ventricular apex via the right or left femoral vein, respectively. A decapolar catheter was advanced into the LV via the left femoral artery. If the clinical arrhythmias did not occur spontaneously, isoproterenol (3 to 5 µg/minute) was intravenously administered. Induction of the VT or PVCs was attempted by programmed ventricular stimulation from the right ventricular apex, with up to three extra-stimuli introduced after an eight-beat baseline ventricular pacing. During the mapping and ablation in the LV, intravenous heparin was administered to maintain an activated clotting time >250 seconds.
Activation and pace mapping were performed in all patients to identify ablation target locations. In patients who had infrequent PVCs, pace mapping was guided to identify the site of origin of VAs. Perfect pace maps were defined as full matching (12/12 leads).
We defined Purkinje potentials, shown as pre-systolic, high-frequency potentials recorded continuously from the LV inferobasal septum to the inferior midseptal segment using a decapolar catheter during sinus rhythm.
In two of 12 patients, a three-dimensional electroanatomical mapping system was used to guide mapping. One of these patients underwent PVC ablation using a non-contact mapping system (EnSite Array, St. Jude Medical, St. Paul, MN, USA), and NavX (St. Jude Medical, Minnetonka, MN, USA) was used in the other.
Radiofrequency catheter ablation was performed with a quadripolar deflectable 3.5-mm irrigated tip ablation catheter (Navistar Thermocool, Biosense Webster, Diamond Bar, CA, USA) in nine patients. With an irrigated catheter, the power was set to 30-40 W with an irrigation flow rate of 17-25 mL/minute, and the maximal temperature was set to ≤48℃. Non-irrigated radiofrequency (RF) current with a 5-mm tip ablation catheter was delivered with a target temperature of 60℃ and a maximum power output of 50 W. When an acceleration or disappearance of VT or PVCs was observed during the first 30 seconds of the application, the RF current was delivered for an additional 60-120 seconds; otherwise, the RF delivery was terminated, and the catheter was repositioned.
During or after ablation around the PMs, precise anatomic positioning of the ablation catheter was confirmed using transthoracic, transesophageal or intracardiac echocardiography (ICE) (Vivid-Seven, General Electric Vingmed, Horten, Norway) and left ventriculography.
The acute success of RFCA was defined as the complete elimination and non-inducibility of VT or PVCs, both in the absence and presence of isoproterenol infusion (3-5 µg/minute) and by burst ventricular pacing.
Local electrogram analysis was retrospectively performed to distinguish the electrogram characteristics at the successful ablation sites of the VAs arising from the LV PM. The analysis focused on the following: 1) the presence of the distal bipolar electrogram from the onset of the surface QRS, 2) the R wave amplitude and duration on the distal bipolar electrogram during VA, 3) the morphology of the initial component of the distal unipolar electrogram, calculated as the time (Δt) from the initial QS wave onset to the initial QS wave peak on the unipolar electrogram.
All patients underwent 24-hour telemetry monitoring immediately after the procedure. Antiarrhythmic medications were discontinued if RFCA was acutely successful. Patients were seen in the outpatient clinic every three months following the procedure for recurrence assessment of VAs. During follow-up, 12-lead surface ECG and 24-hour Holter monitoring were performed in all patients. We considered successful catheter ablation as no recurrence of symptomatic VAs in the absence of any antiarrhythmic drugs. Regarding recurrent PVCs, PVC burden was evaluated before and after the procedure.
All continuous variables are expressed as the mean±standard deviation, and all categorical variables are presented as number and percentage. For continuous variables, the comparisons were performed using Student's t-test. Categorical variables were compared using the Fisher's exact test or chi-square analysis as appropriate. If a cell size was <5, Fisher's exact test was used. A p<0.05 was considered statistically significant.
We determined the cut-off value for the R wave amplitude to differentiate between successful and recurrence groups based on the receiver-operator characteristic curve.
Twelve patients (4.2%) who had a VA originating at the LV PM were identified. General characteristics of the study patients are given in Table 1. Seven of 12 patients were male, and the mean age was 52±9 years (range 37-67 years). The mean duration of symptoms prior to the ablation procedure was 8.6±10.9 months. Symptoms included palpitations in eight patients, dyspnea on exertion in three patients and syncope in one patient. The mean LV ejection fraction (LVEF) was 53±9%. Patients received a mean of 1.2±0.8 antiarrhythmic drugs at the time of the procedure. None of the 12 patients responded to intravenous or oral verapamil. Three patients previously underwent RFCA for clinical VAs at another hospital. The clinically presenting VA was non-sustained VT in six patients (Fig. 1A), sustained VT in four patients and frequent PVCs in the remaining two patients.
The VT or PVCs demonstrated a right bundle branch block with left superior axis QRS morphology in ten patients, with right superior axis in one and inferior axis in one patient. The mean QRS duration during VAs was 146±17 ms. The notch of the R wave in more than three consecutive precordial leads (Fig. 1B) was present in eight patients with VAs originating from LV posterior PM (n=7) and anterior PM (n=1). Of the eight patients with PVCs and non-sustained VT, all had spontaneous PVCs on electrophysiological study. Additionally, four of eight patients demonstrated provocation of PVCs and non-sustained VT following isoproterenol infusion. Of the four patients with sustained VT as the clinical arrhythmia, the cycle length of VT varied between 40 and 80 ms, and VT occurred spontaneously in three patients. In one patient, PVCs with a QRS morphology identical to that of clinical VT, were frequently observed at baseline.
Radiofrequency catheter ablation was performed at the site of the earliest ventricular activation with or without perfectly matched pace mapping. Transthoracic (n=4) or transesophageal echocardiography (n=1), ICE (n=5) and left ventriculography (n=9) revealed that the earliest site of ventricular activation was localized at the base of the anterior PM (n=2), and posterior PM (n=10) in the LV (Fig. 2). In three patients, the QRS morphology of VAs changed into one of two or three different patterns after ablation, and additional ablation around the PM near the first RF lesion was required to completely eliminate the VAs. In all but one patient, successful ablation was achieved using an open-irrigated RF ablation catheter. The acceleration of the VT or PVCs occurred during the RF applications in nine patients (75.0%). In one patient, ventricular fibrillation occurred during RFCA, which required urgent defibrillation using DC. There were no complications related to the procedures.
During the 12±9 months of follow-up (range, 3-23 months), eight patients remained VA-free, while the remaining four patients experienced VA recurrence (33.3% recurrence rate) that occurred at a mean of 50±43 days after the first ablation procedure. Among patients with VA recurrence, one patient received a second RFCA 16 days after the first procedure. This patient remained VT-free after repeat ablation. However, three patients who did not undergo repeat ablation procedure showed significant improvement in symptoms and a substantial reduction in the PVC burden (25±5% to 3±2%) without the use of any antiarrhythmic drugs.
Analysis results of determinants of successful outcome after RFCA is shown in Table 2. The clinical and electrocardiographical characteristics were similar between VAs with and without recurrence. Representative local electrograms of successful and unsuccessful ablation sites in the VAs originating from the PM in the LV are shown in Fig. 3. The distal bipolar electrogram at the successful ablation site exhibited a significantly greater R wave amplitude (1.42±0.55 vs. 0.44±0.23, p=0.01). However, R wave duration was similar between the two groups. There was no Purkinje potential during sinus rhythm in six of eight patients (75.0%). The cut-off R wave amplitude for differentiating between the successful and recurrence groups was 1.0 mV, with a sensitivity/specificity of 85%/80%, although the study number was too small to establish a dependable conclusion. In seven of eight patients, the high-amplitude (>1.0 mV) discrete potential preceded the onset of the QRS complex during VAs (Fig. 3A). The high-amplitude muscle potential preceded the onset of the surface QRS complex by 20-40 ms (mean 33±7 ms). In the remaining four unsuccessful patients, low-amplitude fractionated potentials (Fig. 3B) preceded the onset of the QRS complex during VAs by 23-50 ms (mean 35±9 ms). In two of these four patients, Purkinje potentials during sinus rhythm were observed at the ablation site. There were no differences in symptom duration, QRS width or LVEF in the two subgroups. All but one patient had a QS wave on unipolar electrogram. The mean duration from onset to peak downstroke on unipolar electrogram was 58±8 ms in the successful group and 37±9 ms in the unsuccessful group. A slow downstroke >50 ms of the initial Q wave on the unipolar electrogram was more frequently observed in the successful group than in the recurrence group (85.7% vs. 25.0%, p=0.03).
In VAs originating from the PM in the LV, the high-amplitude discrete potentials before QRS and a slow downstroke of the initial Q wave on the unipolar electrogram at ablation sites were related to favorable outcome.
The role of Purkinje potentials in the reentrant circuit of ILVT was demonstrated in several previous studies.14-16) Recently, Doppalapudi et al.7) reported that, in VAs originating from PM, no high-frequency Purkinje potential were identified at effective ablation sites, suggesting that the Purkinje system is not directly involved. They also demonstrated that, although Purkinje potentials were often recorded during sinus rhythm at the ablation site, there was typically a reversal of Purkinje-ventricular muscle electrograms during PVCs or VT. In contrast, Good et al.8) reported that more distal Purkinje potentials were identified from the Purkinje-myocardial interface located at the PM, and the distal Purkinje system was involved in the development of the LV PM VAs. In the current study, Purkinje potentials were not consistently recorded at the ablation site, suggesting that the Purkinje system may not be a part of the VT or PVC originating from PM. In a previous study,13) the presence of Purkinje potentials at the site of origin was associated with favorable RFCA outcome. In the present study, high-amplitude discrete potentials during VAs did not present as Purkinje potentials during sinus rhythm in six of eight patients, and these potentials may not be associated with the Purkinje activation during sinus rhythm. Therefore, we suggest the high-amplitude discrete potential is developed from arrhythmogenic PM. In four patients with PM VAs, low-amplitude fractionated ventricular muscle potentials were also observed in the ablation site. We speculated that the differences between low and high amplitude potentials might be caused by the proximity of an arrhythmogenic myocardium. High-amplitude potentials might reflect near-field activities of the subendocardial VA origin in the PM, whereas low-amplitude fractionated potentials might reflect far-field activities of the VA origins deep inside the PM. Unfortunately, we did not evaluate the exact proximity of the ablation site or myocardial characteristics using cardiac imaging tools such as cardiac MRI. Additionally, statistical significance between symptom duration, QRS width, LVEF and LV dimension was not observed between the two subgroups.
Further studies to assess the correlation between the precise anatomical site and arrhythmogenic substrate of VAs with the presence or absence of preceding discrete high-amplitude potentials are necessary.
The unipolar electrogram at the successful ablation site mostly exhibited a QS wave in which the initial downstroke of the Q wave had a relatively flat slope, possibly reflecting the slow propagation of a wavefront from the arrhythmogenic myocardial tissue. Therefore, the downstroke pattern on the unipolar electrogram showed significant difference between patients with recurrence and successful outcome.
The electrocardiographic features of VA originating from LV PM are very similar to those of ILVT. However, the spontaneous occurrence of non-sustained VT was commonly demonstrated in patients with VA originating from PM. Additionally, in PM VA patients, a notch of the R wave in three or more precordial leads was often present, in contrast to a previous report.8) The cause of QRS notching in PM VAs remains to be determined. We speculated that the mechanism of notching was associated with the conduction delay through the arrhythmogenic PM into the normal myocardium. This is also related to the broader QRS complex in the PM group compared to fascicular VT because ventricular activation is caused by direct local PM activation apart from the fascicles of the distal Purkinje system or slower conduction to the rest of the ventricle than in ILVT.
In most patients, VAs were not inducible with programmed electrical stimulation. This finding suggests a non-reentrant mechanism for this type of arrhythmia. Furthermore, PVCs were provoked by intravenous isoproterenol, suggesting that the underlying mechanism is triggered activity or abnormally enhanced automaticity. Acceleration of VT or PVCs occurred during RF application in nine patients. This also suggests that abnormal automaticity may be the mechanism of VA originating from PM.17)
The diagnosis of PM VA was mainly identified according to anatomic site of successful ablation on various cardiac imaging techniques including transthoracic or transesophageal echocardiography, ICE and ventriculography. However, these diagnostic tools were limited in determining the exact catheter tip location even when using ICE.
This was a retrospective study with a small number of patients; therefore, further prospective studies with a larger number of patients are warranted to draw more definitive conclusions.
Figures and Tables
 | Fig. 1The 12-lead ECG of ventricular arrhythmias originating from the posterior papillary muscle in the left ventricle. A: the representative 12-lead ECG of a non-sustained VT originating from the LV PPM. B: notches in precordial leads during VAs originating from the LV PPM (left panel) and LV APM (right panel). Arrows indicate notches in the precordial leads. ECG: electrocardiogram, VT: ventricular tachycardia, LV: left ventricle, PPM: posterior papillary muscle, VAs: ventricular arrhythmias, APM: anterior papillary muscle. |
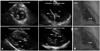 | Fig. 2The echocardiographic and fluoroscopic images exhibiting the successful ablation site. A: the echocardiographic image of the successful ablation site. A two-dimensional transthoracic echocardiographic (left panel) and intra-cardiac echocardiographic (right panel) image at the level of the PM demonstrated that the ablation catheter was positioned on the base of the PPM. Transthoracic echocardiography showed the exact ablation catheter laid at the PPM in the parasternal short axis and long axis view, respectively (left panel). The anterior and posterior short axis view intra-cardiac echocardiography showed the ablation catheter on the base of PPM in the LV (right panel). The arrow indicates the ablation catheter. B: right anterior oblique fluoroscopic image of the successful ablation site referenced to left ventriculograms. The small arrows indicate the border of the PPM during left ventricular systole (upper panel) and diastole (lower panel). ABL: ablation catheter, LV: left ventricle, PM: papillary muscle, PPM: posterior papillary muscle, RAO: right anterior oblique view. |
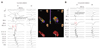 | Fig. 3Local bipolar electrogram, unipolar electrogram and three-dimensional electroanatomic mapping images of successful (A) and unsuccessful (B) ablation sites of VT originating from the PPM in the LV. A: a high-amplitude discrete potential (arrow) from the distal bipole of the ablation catheter was recorded during VT (left panel) and Purkinje potential (asterisk) during sinus rhythm (middle panel) at the site of successful ablation. A relatively slow downstroke of initial Q wave on unipolar electrogram was observed. The duration from onset to peak downstroke was 62 ms. The three-dimensional electroanatomical mapping (right panel) obtained during procedure in the right and left anterior oblique view showed the color-coded voltage map (right upper panel) and successful ablation site (right lower panel) of the PPM in the LV. White dots represent the site of RFCA. White arrows indicate the PPM area in the LV. B: a low-amplitude myocardial potential (arrow) recorded during VT from the distal bipole of the ablation catheter preceded the onset of QRS by 34 ms. A relatively steep downstroke of initial Q wave on unipolar electrogram was observed at the unsuccessful ablation site (left panel). The duration from onset to peak downstroke was 42 ms. No Purkinje potential was shown during sinus rhythm (right panel). ABL: ablation catheter, LAO: left anterior oblique view, LV: left ventricle, PPM: posterior papillary muscle, RAO: right anterior oblique view, RVA: right ventricular apex, SR: sinus rhythm, VT: ventricular tachycardia, RFCA: radiofrequency catheter ablation, Δt: the time from the initial QS wave onset to the initial QS wave peak on unipolar electrogram. |
References
1. Kim YH, Xie F, Yashima M, et al. Role of papillary muscle in the generation and maintenance of reentry during ventricular tachycardia and fibrillation in isolated swine right ventricle. Circulation. 1999; 100:1450–1459.
2. Pak HN, Kim YH, Lim HE, et al. Role of the posterior papillary muscle and purkinje potentials in the mechanism of ventricular fibrillation in open chest dogs and Swine: effects of catheter ablation. J Cardiovasc Electrophysiol. 2006; 17:777–783.
3. Chen PS, Karagueuzian HS, Kim YH. Papillary muscle hypothesis of idiopathic left ventricular tachycardia. J Am Coll Cardiol. 2001; 37:1475–1476.
4. Yamada T, Tabereaux PB, Doppalapudi H, McElderry HT, Kay GN. Successful catheter ablation of a ventricular tachycardia storm originating from the left ventricular posterior papillary muscle involved with a remote myocardial infarction. J Interv Card Electrophysiol. 2009; 24:143–145.
5. Liu XK, Barrett R, Packer DL, Asirvatham SJ. Successful management of recurrent ventricular tachycardia by electrical isolation of anterolateral papillary muscle. Heart Rhythm. 2008; 5:479–482.
6. Bogun F, Desjardins B, Crawford T, et al. Post-infarction ventricular arrhythmias originating in papillary muscles. J Am Coll Cardiol. 2008; 51:1794–1802.
7. Doppalapudi H, Yamada T, McElderry HT, Plumb VJ, Epstein AE, Kay GN. Ventricular tachycardia originating from the posterior papillary muscle in the left ventricle: a distinct clinical syndrome. Circ Arrhythm Electrophysiol. 2008; 1:23–29.
8. Good E, Desjardins B, Jongnarangsin K, et al. Ventricular arrhythmias originating from a papillary muscle in patients without prior infarction: a comparison with fascicular arrhythmias. Heart Rhythm. 2008; 5:1530–1537.
9. Yamada T, McElderry HT, Okada T, et al. Idiopathic focal ventricular arrhythmias originating from the anterior papillary muscle in the left ventricle. J Cardiovasc Electrophysiol. 2009; 20:866–872.
10. Yamada T, Doppalapudi H, McElderry HT, et al. Idiopathic ventricular arrhythmias originating from the papillary muscles in the left ventricle: prevalence, electrocardiographic and electrophysiological characteristics, and results of the radiofrequency catheter ablation. J Cardiovasc Electrophysiol. 2010; 21:62–69.
11. Yamada T, Doppalapudi H, McElderry HT, et al. Electrocardiographic and electrophysiological characteristics in idiopathic ventricular arrhythmias originating from the papillary muscles in the left ventricle: relevance for catheter ablation. Circ Arrhythm Electrophysiol. 2010; 3:324–331.
12. Crawford T, Mueller G, Good E, et al. Ventricular arrhythmias originating from papillary muscles in the right ventricle. Heart Rhythm. 2010; 7:725–730.
13. Yokokawa M, Good E, Desjardins B, et al. Predictors of successful catheter ablation of ventricular arrhythmias arising from the papillary muscles. Heart Rhythm. 2010; 7:1654–1659.
14. Nogami A, Naito S, Tada H, et al. Demonstration of diastolic and presystolic Purkinje potentials as critical potentials in a macroreentry circuit of verapamil-sensitive idiopathic left ventricular tachycardia. J Am Coll Cardiol. 2000; 36:811–823.
15. Aiba T, Suyama K, Aihara N, et al. The role of Purkinje and pre-Purkinje potentials in the reentrant circuit of verapamil-sensitive idiopathic LV tachycardia. Pacing Clin Electrophysiol. 2001; 24:333–344.
16. Maruyama M, Tadera T, Miyamoto S, Ino T. Demonstration of the reentrant circuit of verapamil-sensitive idiopathic left ventricular tachycardia: direct evidence for macroreentry as the underlying mechanism. J Cardiovasc Electrophysiol. 2001; 12:968–972.
17. Zipes DP. Mechanisms of clinical arrhythmias. J Cardiovasc Electrophysiol. 2003; 14:902–912.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download