Abstract
The ductus arteriosus is a normal and essential structure in fetal circulation. Since the introduction of fetal echocardiography, there have been reports of ductal constriction, many of which were related to maternal use of some medications. However, there have been some reports of idiopathic ductal constriction, which usually present in later gestation. Recently we experienced a case, which initially showed an S-shaped ductus with mild narrowing at 23 weeks and 27 weeks gestation and developed severe ductal constriction at 33 weeks. Soon after birth, ductus was searched for but no ductus was found in 2-D and color images. The neonate required mechanical ventilation with supplemental oxygen for 3 days. All echocardiographic abnormalities were normalized in 7 months. We report progressive ductal constriction in an S-shaped ductus and emphasize the importance of continuous follow up extending to the third trimester and even immediately after birth.
The ductus arteriosus (DA) is a normal and essential structure in fetal circulation, and carries most of the right ventricular output to the descending aorta. In fetal life, ductal patency is actively maintained by factors such as prostaglandin E2 produced in the placenta, endothelial nitric oxide (NO) synthase and low oxygen tension of the blood.
Since the introduction of fetal echocardiography, there have been reports of ductal constriction, many of which are related to maternal use of cyclooxygenase-inhibitors or other medications. However, some ductal constrictions are not related to drugs or other medications and usually present with right ventricular failure and fetal hydrops in the third trimester. Theoretically, ductal constriction causes pressure overload to the right ventricle (RV) and ultimately right ventricular failure. However, symptoms of right ventricular failure may vary from critical symptoms such as fetal hydrops to mild symptoms. This variability is probably related to the severity of ductal constriction, but other possible factors could be gestational age at development, rapidity of constriction development, presence or absence of tricuspid regurgitation (TR) and flow amount through the right side of the heart.
Recently, we experienced a case which showed progression from mild to severe ductal constriction some time between 27 and 33 weeks and would like to emphasize the importance of extended follow up examination until delivery.
A 22-year-old woman, gravid 2, para 1-0-0-1, was referred to our institution for fetal echocardiography at 23 weeks of gestation because of abnormally looking great arterial arches. The pregnancy had not been complicated. The mother had not had any diseases or taken any drugs or herbal medications. There was no family history of congenital heart disease. Obstetric evaluation showed normal fetal growth, normal amount of amniotic fluid and normal placenta. The fetal echocardiography revealed the normal four chamber view, where two atria and two ventricles were normal in size and thickness (Fig. 1A). The two atrioventricular valves moved normally. Two great arteries arose normally from the appropriate ventricles. The pulmonary and aortic valves looked normal and the size of the main pulmonary artery and aortic root was within normal limits. The aortic arch was normal in size and in location, but the ductal arch was not visualized in any views. The main pulmonary artery bifurcated into two branch pulmonary arteries but was not continuous with the DA (Fig. 1B). However color Doppler examination showed a tortuous 'S' shaped ductus cuddled inside the aortic arch (Fig. 1C and D). The DA joined the descending aorta with an obtuse angle and showed a flow signal from the main pulmonary artery to the descending aorta (Fig. 1E and F). Color aliasing occurred along the course of the ductus but pulsed Doppler measurement of velocity was not attempted because of the poor angle of insonation. Flow velocity and waveforms of the two great arteries were within normal limits and no TR was found. A diagnosis of S-shaped DA with mild narrowing was made and follow up examination was arranged.
A repeat examination was performed at 27 weeks of gestation and showed nearly the same findings as before. The RV was normal in size and thickness and no TR was noted. Because the DA was S-shaped, it was very difficult to assess size along the whole range of the DA. However, the color Doppler examination showed tortuous turbulent flow in the ductus and suggested mild obstruction. The sagittal section of the aortic arch showed a ductal junction through which low velocity forward flow from the DA to the descending aorta was noted. Obstetric evaluation showed that fetal growth was appropriate and the amniotic fluid index was adequate. No signs of hydrops were noted.
The third examination was performed at 33 weeks of gestation. The right atrium was big and the RV was hypertrophied and globular in shape. The intracavitary volume of the RV looked slightly smaller than normal because of the thick ventricular wall and hypertrophy of the trabeculations. The right ventricular endocardial lining and the chordae of the tricuspid valve were very bright (Fig. 2A). Color Doppler examination showed moderate TR and its velocity was 4.5 m/sec (Fig. 2B and C). Every attempt failed to demonstrate any flow through the ductus. In spite of the right ventricular overload, fetal growth was within normal range and there was no evidence of fetal hydrops.
After a discussion, we decided to deliver the baby. A female infant weighing 2625 g was born at 34 weeks gestation by induced delivery. Apgar scores were 7 at 1 minute and 8 at 5 minutes. Symptoms and signs immediately after birth were tachypnea, chest retraction, grunting sound and low oxygen saturation. An echocardiography performed at 20 minutes after birth showed a small hypertrophied RV and increased echogenicity of the moderator band and chordae of tricuspid valve (Fig. 3A). There was a moderate TR with a velocity of 3.7 m/sec (Fig. 3B and C). The short axis view showed a hypertrophied RV (Fig. 3D). The ductus was searched for but no ductus was found in 2-D and color images (Fig. 3E). Shunt flow through the foramen ovale was mainly right to left (Fig. 3F). She required respiratory support with a mechanical ventilator for the first 3 days. She was successfully weaned from the ventilator on her fourth day and discharged at 12 days old. Follow-up examination at the outpatient clinic showed that she did not have any cardiac symptoms or signs. Echocardiography taken at 2 months of age showed much improvement. The RV was nearly normal in size and thickness. Increased echogenicity of the RV was also nearly normalized. There remained TR with a velocity of 3.0 m/sec. At 7 months of age, all echocardiographic findings returned to normal with resolution of TR.
Since the introduction of fetal echocardiography, various abnormalities of the DA have been described.1) Among ductal diseases in the fetus, ductal constriction or premature closure is one of the most important diseases because it is associated with significant morbidity or mortality. Traditionally, ductal constriction is defined as a systolic velocity higher than 1.4 m/s and a diastolic velocity higher than 0.3 m/s in conjunction with a pulsatility index smaller than 1.9.2)3) This criterion is based on clinical observations in which normal fetuses were compared to a ductal constriction group and a terbutaline group.2) In their study, the number of normal fetuses was only 41 and Doppler waveforms were obtained by the continuous Doppler technique. Mielke and Benda3) measured ductal velocity by pulsed Doppler technique in normal fetuses and showed that the 95 percentile of systolic velocity was well above 1.4 m/s in later gestation. Ductal constriction literally means that the ductal luminal diameter is smaller than normal. However, ductal constriction is usually defined by Doppler velocity4-8) due to difficulties of measuring the luminal diameter. Measurement of the dimensions is more difficult when the ductus is S-shaped or pig-tail shaped. Nonetheless Doppler velocity is not without fault. Not to mention the technical difficulties of obtaining a Doppler spectrum from fetal small vessels, Doppler velocity is also flow dependent. The pulsatility index might be useful to differentiate ductal constriction from increased flow. However, in cases with decreased flow, ductal velocity could be normal in the presence of severe constriction.
In order to assess ductal constriction in a more comprehensible way, the estimated pressure of the RV, flow through the right side of heart and the response of the RV to pressure overload should be added. Hemodynamically significant ductal constriction makes the systemic and pulmonary circulation independent and right ventricular pressure becomes higher than left ventricular pressure when right sided flow is maintained. However, right ventricular pressure may not be high if flow is decreased. Right ventricular pressure and flow through the main pulmonary artery can be measured by the Doppler technique. Diameter and flow of the main pulmonary artery can be compared to those of the aorta. The fetal RV seems to respond to pressure overload in two ways, one is a strain pattern and the other is a failure pattern. In RV strain, the RV is hypertrophied and the endocardium becomes echogenic. The right ventricular cavity is normal or slightly decreased. This case corresponds to this category. Till the 2nd trimester, we could not find any evidence of RV dysfunction. However, in the 3rd trimester and the immediate post natal period, we deduced RV diastolic dysfunction from the echocardiographic images. In contrast, the RV failure pattern shows a dilated and poorly contracting ventricle. Some of the failure group might have increased echogenicity. Premature closure of the ductus is diagnosed when there is no luminal patency and no flow through the ductus. Premature closure should be differentiated from absence of the ductus.1) Ductal absence is thought to be an early developmental anomaly and is associated with certain cardiac anomalies such as absent pulmonary valve syndrome and truncus arteriosus.
Fetal ductal constriction or premature closure is mostly associated with the use of cyclooxygenase-inhibiting drugs. Recently, other drugs and special foods have also been associated with ductal constriction.9) However, the idiopathic cases of fetal ductal constriction are few.10-13) Our case was diagnosed to have idiopathic severe ductal constriction or premature closure at 33 weeks and it was associated with suprasystemic RV pressure and RV strain. However, previous two examinations at 23 and 27 weeks gestation showed only an S-shaped ductus with possible mild narrowing but without any right ventricular abnormalities. Our case is very similar to a case reported by Trevitt and Cotton.4) That case also showed an S-shaped ductus at 27 weeks without any cardiac abnormalities but severe ductal constriction with RV strain developed at 33 weeks. These two cases certainly prove that DA may develop progressive dense calcified in the 3rd trimester.
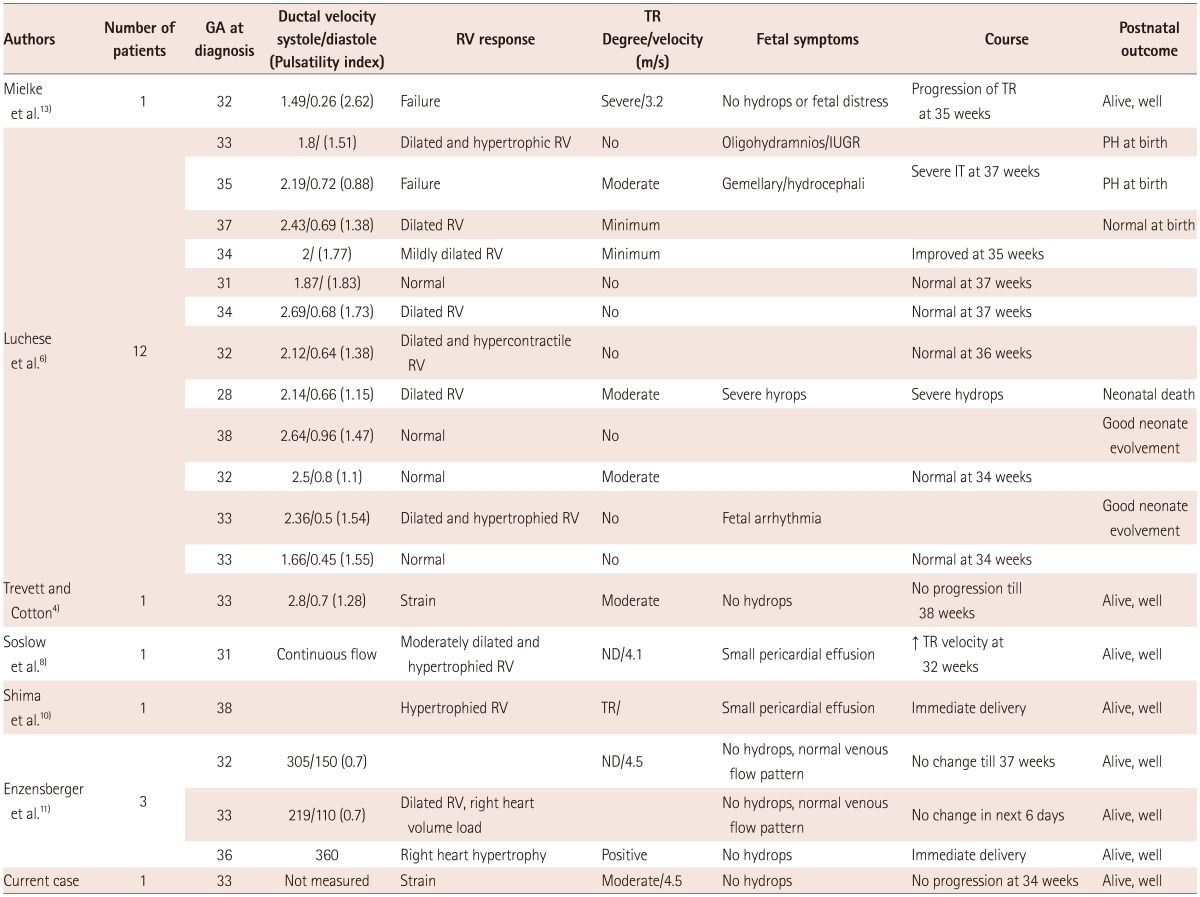
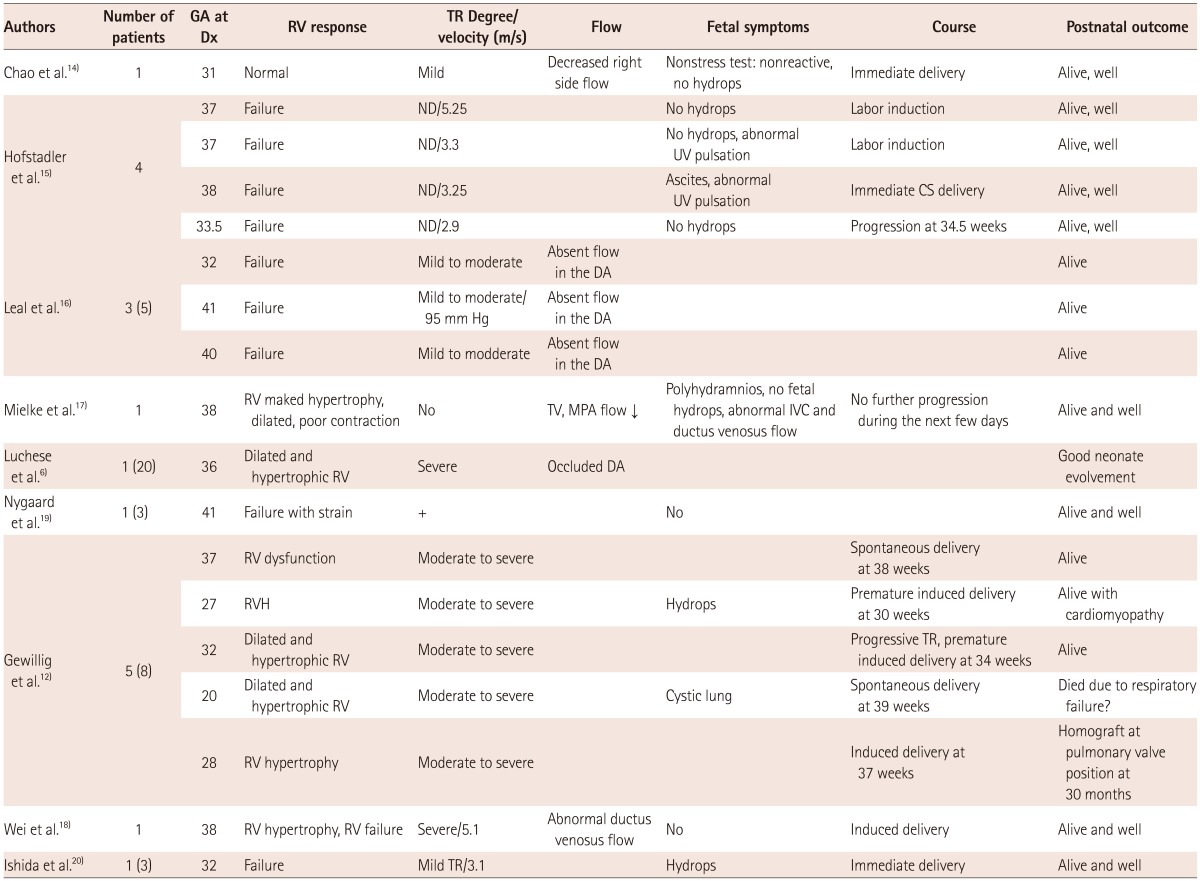
In order to review idiopathic ductal constriction or premature closure, a literature search was performed in PubMed and PubMed Central. In some reports, both idiopathic cases and drug-related constriction and premature closure were included.6) All idiopathic cases were selected and were divided into a constriction group or a premature closure group and tabulated in Table 1 and 2.
There have been 20 cases of idiopathic ductal constriction including our case (Table 1). Luchese et al.6) reported 13 cases of idiopathic ductal constriction, and used traditional diagnostic criteria for inclusion. Their cases seemed to include ductal constriction of variable degree, from mild to severe, and from asymptomatic to symptomatic. However all others are reports of symptomatic cases. The diagnosis of symptomatic ductal constriction was usually made between 28 and 38 weeks, and mostly after 31 weeks. Significant TR was a commonly associated finding. The RV response was either failure or a strain pattern. Among 30 cases of idiopathic premature closure of the DA (Table 2),14-20) two cases in Gewilling's report12) seemed to have ductal absence rather than premature closure. The diagnosis of premature closure is usually made between 28-41 weeks. Fetal symptoms, degree of TR, and the right ventricular response in cases with premature closure seem to be similar to those with severe symptomatic ductal constriction. However, the velocity of TR was more variable and ranged from 2.9 to 5.25 m/sec, probably depending on the flow through the right heart and contractility of the RV, and degree of TR.
With regard to management, fetuses with right ventricular failure and other signs of fetal distress such as hydrops are usually managed by immediate delivery if the fetuses are not too premature. However, if the fetuses are too premature, a decision on delivery should balance fetal risk and post-natal risk. The fetus without fetal distress can be followed under careful observation. Pulmonary hypertension and right ventricular failure are major concerns after delivery, but the post-natal prognosis seems to be good. Severely affected babies require supplemental oxygen or mechanical ventilation. Pulmonary hypertension can be managed with NO. Right heart abnormalities seem to resolve within a couple of weeks, but right ventricular wall thickness and diastolic dysfunction take several months to recover.
In summary, we report a case in which a fetal S-shaped ductus with mild narrowing at 23 and 27 weeks gestation showed progressive ductal constriction or premature closure at 33 weeks, and emphasize the importance of follow up well into the 3rd trimester. It is also suggested that the diagnosis of ductal constriction should be based on not only the ductal diameter, flow velocity, and pulsatility index, but also on right ventricular pressure, flow through right side of the heart and the right ventricular response to pressure overload.
Acknowledgments
The Institutional Review Board of the Seoul National Bundang Hospital approved this report.
References
1. Weichert J, Hartge DR, Axt-Fliedner R. The fetal ductus arteriosus and its abnormalities--a review. Congenit Heart Dis. 2010; 5:398–408. PMID: 21087423.
2. Tulzer G, Gudmundsson S, Sharkey AM, Wood DC, Cohen AW, Huhta JC. Doppler echocardiography of fetal ductus arteriosus constriction versus increased right ventricular output. J Am Coll Cardiol. 1991; 18:532–536. PMID: 1856423.
3. Mielke G, Benda N. Blood flow velocity waveforms of the fetal pulmonary artery and the ductus arteriosus: reference ranges from 13 weeks to term. Ultrasound Obstet Gynecol. 2000; 15:213–218. PMID: 10846777.
4. Trevett TN Jr, Cotton J. Idiopathic constriction of the fetal ductus arteriosus. Ultrasound Obstet Gynecol. 2004; 23:517–519. PMID: 15133807.
5. Harada K, Rice MJ, Shiota T, McDonald RW, Reller MD, Sahn DJ. Two-dimensional echocardiographic evaluation of ventricular systolic function in human fetuses with ductal constriction. Ultrasound Obstet Gynecol. 1997; 10:247–253. PMID: 9383875.
6. Luchese S, Mânica JL, Zielinsky P. Intrauterine ductus arteriosus constriction: analysis of a historic cohort of 20 cases. Arq Bras Cardiol. 2003; 81:405–410. 399–404. PMID: 14666282.
7. Benson CB, Brown DL, Doubilet PM, DiSalvo DN, Laing FC, Frates MC. Increasing curvature of the normal fetal ductus arteriosus with advancing gestational age. Ultrasound Obstet Gynecol. 1995; 5:95–97. PMID: 7719875.
8. Soslow JH, Friedberg MK, Silverman NH. Idiopathic premature closure of the ductus arteriosus: an indication for early delivery. Echocardiography. 2008; 25:650–652. PMID: 18422673.
9. Zielinsky P, Piccoli AL Jr, Manica JL, et al. Maternal consumption of polyphenol-rich foods in late pregnancy and fetal ductus arteriosus flow dynamics. J Perinatol. 2010; 30:17–21. PMID: 19641513.
10. Shima Y, Ishikawa H, Matsumura Y, Yashiro K, Nakajima M, Migita M. Idiopathic severe constriction of the fetal ductus arteriosus: a possible underestimated pathophysiology. Eur J Pediatr. 2011; 170:237–240. PMID: 20845046.
11. Enzensberger C, Wienhard J, Weichert J, et al. Idiopathic constriction of the fetal ductus arteriosus: three cases and review of the literature. J Ultrasound Med. 2012; 31:1285–1291. PMID: 22837295.
12. Gewillig M, Brown SC, De Catte L, et al. Premature foetal closure of the arterial duct: clinical presentations and outcome. Eur Heart J. 2009; 30:1530–1536. PMID: 19389789.
13. Mielke G, Peukert U, Krapp M, Schneider-Pungs J, Gembruch U. Fetal and transient neonatal right heart dilatation with severe tricuspid valve insufficiency in association with abnormally S-shaped kinking of the ductus arteriosus. Ultrasound Obstet Gynecol. 1995; 5:338–341. PMID: 7614140.
14. Chao RC, Ho ES, Hsieh KS. Doppler echocardiographic diagnosis of intrauterine closure of the ductus arteriosus. Prenat Diagn. 1993; 13:989–994. PMID: 8309905.
15. Hofstadler G, Tulzer G, Altmann R, Schmitt K, Danford D, Huhta JC. Spontaneous closure of the human fetal ductus arteriosus--A cause of fetal congestive heart failure. Am J Obstet Gynecol. 1996; 174:879–883. PMID: 8633660.
16. Leal SD, Cavallé-Garrido T, Ryan G, Farine D, Heilbut M, Smallhorn JF. Isolated ductal closure in utero diagnosed by fetal echocardiography. Am J Perinatol. 1997; 14:205–210. PMID: 9259929.
17. Mielke G, Steil E, Breuer J, Goelz R. Circulatory changes following intrauterine closure of the ductus arteriosus in the human fetus and newborn. Prenat Diagn. 1998; 18:139–145. PMID: 9516015.
18. Wei S, Ailu C, Ying Z, Yili Z. Idiopathic occlusion of the fetal ductus arteriosus without lumen narrowing. Echocardiography. 2011; 28:E85–E88. PMID: 21426387.
19. Nygaard SI, Petersen OB, Garne E, Sørensen KE. Spontaneous prenatal ductal closure: postnatal diagnosis? Pediatr Cardiol. 2009; 30:176–180. PMID: 18779991.
20. Ishida H, Inamura N, Kawazu Y, Kayatani F. Clinical features of the complete closure of the ductus arteriosus prenatally. Congenit Heart Dis. 2011; 6:51–56. PMID: 21269413.
Fig. 1
The fetal echocardiography was done at 23 weeks of gestation. A: the four chamber view shows normal ventricular size and wall thickness. B: aortic arch was normal in size and location, but the ductal arch which connected with the main pulmonary artery (MPA) was not visualized. C: the main pulmonary artery bifurcated into the left pulmonary artery (LPA) and the right pulmonary artery (RPA) but it had no visible continuity with the ductus arteriosus (DA). D: only the color Doppler examination showed the tortuous S shaped DA (arrow). E: the ductus joined the descending aorta at an obtuse angle. F: color aliasing occurred through the DA (arrow).

Fig. 2
Follow-up echocardiography at 27 weeks showed significant changes in right ventricular morphology and function. A: the right ventricle had become small and thick and its endocardial lining was very bright. B: color Doppler examination showed a moderate amount of tricuspid regurgitation (TR). C: measured TR velocity was 4.5 m/sec.
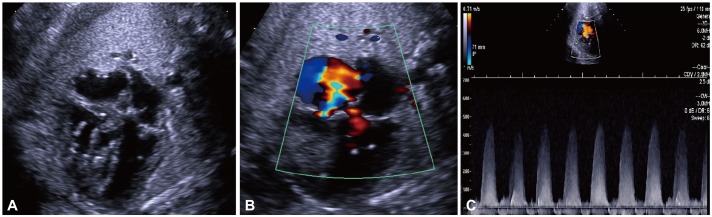
Fig. 3
Post-natal echocardiography was performed within few hours after birth. A: four chamber view showed a hypertrophied RV and hyperechoic chordae of the tricuspid valve. B: the patient had a moderate amount of TR. C: TR velocity was slightly decreased compared to the previous examination during fetal life. D: hypertrophied RV and moderator band are apparent in the short axis view. E: there was no visible flow in the ductus arteriosus. F: shunt direction through the foramen ovale was mainly right to left (arrow). RV: right ventricle, TR: tricuspid regurgitation.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download