Abstract
Background and Objectives
Cardiac involvement is frequent in systemic amyloidosis and is the most important determinant of the clinical outcome. The aims of this study were to assess the incidence and prognosis of cardiac amyloidosis and discuss the diagnostic issues related to cardiac amyloidosis.
Subjects and Methods
We retrospectively studied all patients diagnosed with systemic amyloidosis who presented to our institution from January 1999 to December 2011.
Results
Of the 129 patients with systemic amyloidosis, cardiac amyloidosis was diagnosed in 62 patients. At the 3 years' follow-up of the patients with systemic amyloidosis, there was a statistically significant difference in mortality between patients with cardiac amyloidosis and the rest of the patients (58.1% vs. 37.3%, p=0.008). In the Cox proportional hazard model, old age {hazard ratio (HR) 18.336, p=0.006}, elevation of cardiac troponin I (cTNI) (HR 13.246, p=0.020), left ventricular (LV) systolic dysfunction (HR 5.137, p=0.041) and diastolic dysfunction (HR 64.595, p=0.022) were independently associated with survival in cardiac amyloidosis. In the diagnosis of monoclonal gammopathy, serum or urine protein electrophoresis was not sensitive enough to be used clinically compared to serum free light chain assay (35.8% vs. 96.4%).
Amyloidosis is a clinical disorder caused by the extracellular deposition of insoluble abnormal fibrils, derived from the aggregation of misfolded normally soluble proteins.1) Many different proteins can form amyloid fibrils, and the types of amyloidosis are classified on the basis of the amyloidogenic protein as well as by the distribution of the amyloid deposits, as either systemic or localized.2) In systemic amyloidosis, amyloid deposits are present in the viscera, blood vessel walls, and connective tissues. In contrast, in localized disease, the deposits are confined to specific foci or to a particular organ or tissue.
The heart is not infrequently involved in systemic amyloidosis. However, the diagnosis of cardiac involvement is not easy, and it is frequently underdiagnosed. Making an early diagnosis of cardiac amyloidosis is critical because, once clinically significant heart disease appears, the prognosis is extremely poor.
The gold standard test for the diagnosis of cardiac amyloidosis is endomyocardial biopsy,3) but it cannot be performed in every patient because of its invasiveness. Thus, in clinical practice, the diagnosis of cardiac amyloidosis is often made in patients with systemic amyloidosis when the echocardiographic findings suggest infiltrative cardiomyopathy.4) Recently, cardiovascular magnetic resonance imaging (CMR) with late gadolinium enhancement (LGE) sequence was shown to be useful for the diagnosis of cardiac involvement in systemic amyloidosis.5)6)
In the present study, we assess the incidence and prognosis of cardiac amyloidosis and discuss diagnostic issues related to cardiac amyloidosis.
We retrospectively studied all patients diagnosed as having systemic amyloidosis who presented to the Seoul National University Hospital, Korea, from January 1999 to December 2011. This study was approved by the institutional review board at our institution. Patients were followed up and evaluated for the development of clinical events, using their electronic medical records.
For the detection of the presence of monoclonal gammopathy, protein electrophoresis (PEP) for the presence of M protein or immunoelectrophoresis/immunofixation electrophoresis (IFE/IEP), and free light chain (FLC) assay in serum and/or urine were used. The diagnosis of systemic amyloidosis was based on the histologic confirmation of tissue deposition of amyloid. At the time of the histologic confirmation, specimens were stained with Congo red and the diagnosis of amyloidosis was made when the deposition of amorphous material, which showed apple-green birefringence under a polarized microscope, was detected.7) Immunohistochemical stains using antibodies directed against the serum amyloid P component, transthyretin (TTR), kappa and lambda light chains, and serum amyloid A were performed simultaneously.
The diagnosis of cardiac amyloidosis was made when 1) amyloid deposition was demonstrated in the myocardium by the endomyocardial biopsy, or 2) cardiac involvement on either the echocardiography or CMR was suggested in a patient with systemic amyloidosis. Echocardiography was performed in all patients with systemic amyloidosis. In the echocardiographic findings, cardiac involvement was suggested when the mean left ventricular (LV) thickness was >12 mm (average of end-diastolic septal and inferolateral walls in the parasternal long-axis view)8) and standard 12-lead electrocardiograms (ECG) showed low voltage QRS (defined as a QRS amplitude <0.5 mV in all limb leads). When CMR was performed, cardiac involvement was suggested when there was global, subendocardial or patch LGE of the myocardium.
Cardiac troponin-I (cTNI, normal reference values: <0.5 ng/mL), B-type natriuretic peptide (BNP, measured by chemiluminescent immunometric assay, normal reference values: ≤100 pg/mL) or protype B-type natriuretic peptide (pro-BNP, measured by electrochemiluminescent immunometric assay, normal reference values: 0-84 pg/mL for men under 50 years, 0-194 pg/mL for men above 50 years, 0-155 pg/mL for women under 50 years, 0-222 pg/mL for women above 50 years) were measured.
The 10th International Symposium on Amyloid and Amyloidosis was held 18-22 April 2004, in Tours, France. We adopted these guidelines for the diagnosis of organ involvement in amyloidosis.8)
Data analysis was performed using Statistical Package for the Social Sciences (SPSS) version 17.0 (SPSS Inc., Chicago, IL, USA). Discrete data were summarized as frequencies, while continuous variables were shown as the mean±SD. The chi-square or Fisher's exact test were used for the comparison of categorical variables. The Kolmogorov-Smirnov test was used for the normality test. Student's t-test was used for the comparison of normally distributed continuous variables. In the case of a non-normal distribution, the Mann-Whitney U test and Wilcoxon signed-rank test were used. Survival estimates and cumulative event rates were estimated using the Kaplan-Meier method. A multivariate Cox proportional-hazards regression model was used to find the independent predictors of survival. Factors entered into the multivariate model included those with p<0.10 from the univariate analysis and variables with known prognostic value. A p of <0.05 was considered statistically significant.
Baseline characteristics of the patients with systemic amyloidosis and cardiac amyloidosis are summarized in Table 1 and 2. From January 1999 to December 2011, 129 patients were newly diagnosed with systemic amyloidosis. Among them, 76 patients (58.9%) were male, and the mean age was 57.2±11.5. Hypertension was found in 34 patients (26.4%), diabetes mellitus in 11 (8.5%), dyslipidemia in 7 (5.4%), chronic kidney disease (CKD) in 25 (19.4%) and a smoking history in 2 (1.6%). Cardiac involvement was found in 62 patients (48.1%) and the kidney was the second most commonly involved organ (n=49, 40.0%).
Baseline characteristics were not different between patients with cardiac involvement (n=62) and without cardiac involvement (n=67) except for age and CKD. Patients with cardiac involvement were older and had less CKD than patients without cardiac involvement (60.1±9.7 vs. 54.5±12.4, p=0.006; 8.1% vs. 29.9%, p=0.002). The mean follow-up periods were 759.8±957.8 days vs. 1103.6±1069.5days (p=0.057) in patients with and without cardiac involvement, respectively.
Table 3 shows the characteristics of the patients with systemic amyloidosis (n=129). Light chain (AL) amyloidosis was present in 127 (98.4%) and familial amyloidosis (positive result of TTR gene mutation test) in 2 (1.6%). Among the patients with AL amyloidosis, 32 cases (25.2%) were of the kappa light chain type, and 75 cases (59.1%) were of the lambda light chain type. The presence of monoclonal gammopathy was noted in the serum or urine PEP in 43 of 120 patients (35.8%), serum or urine IEP/IFE in 43 of 103 (41.7%), and serum FLC assay in 81 of 84 (96.4%).
Laboratory, ECG, echocardiographic and CMR findings in cardiac amyloidosis are shown in detail in Table 4. In patients with cardiac amyloidosis (n=62), AL amyloidosis was present in 60 (96.8%) and familial amyloidosis in 2 (3.2%). Among the patients with cardiac involvement in AL amyloidosis, 11 cases (19.6%) were of the kappa light chain type and 45 cases (80.4%) were of the lambda light chain type. In the laboratory findings, 5 of 26 patients (19.2%) had elevation of cTNI and 31 of 31 patients (100.0%) had elevation of BNP or pro-BNP. Endomyocardial biopsy was available in 30 patients, and positive pathology compatible with cardiac amyloidosis was present in 29 patients (96.6%). There was low voltage QRS in the ECG of 53 out of 62 patients (85.5%) (Fig. 1). Echocardiography was available in all patients. Among them, increased LV wall thickness was present in 60 patients (96.8%), LV systolic dysfunction {ejection fraction (EF)<50%} in 31 (50.0%), LV diastolic dysfunction in 56 (90.3%) and pericardial effusion in 37 (60.7%) (Fig. 2). CMR was also available in 15 patients and a positive finding was present in 13 (86.7%) (Fig. 3). In detail, 29 patients were diagnosed as having cardiac involvement by endomyocardial biopsy, and among the rest of the patients (n=33), cardiac involvement was suggested by the echocardiographic findings in 29 patients, and both echocardiographic and CMR findings in 4 patients.
At 3 years' follow-up of the patients with systemic amyloidosis, death occurred in 36 cases in patients with cardiac involvement (n=62) and 25 patients without cardiac involvement (n=67). There were statistically significant differences in mortality between the two groups (58.1% vs. 37.3%, p=0.008) (Fig. 4).
Regarding the effect of treatment (chemotherapy, autologous stem cell transplantation and cardiac transplantation) on the prognosis, in patients with systemic amyloidosis, death occurred in 47 cases in the treatment group (n=100) and 14 cases in the no-treatment group (n=29) at the 3 years' follow-up. There was no statistically significant difference in mortality between the two groups (47.0% vs. 48.3%, p=0.504) (Fig. 5). In addition, no significant effect of treatment on the prognosis was also noted in patients with cardiac amyloidosis. Death occurred in 30 cases in the treatment group (n=52) and 6 cases in the no-treatment group (n=10) (57.7% vs. 60.0%, p=0.161) (Fig. 6).
In the Cox proportional hazards model, cardiac involvement {hazard ratio (HR) 1.732, 95% confidence interval (CI) 1.022-2.935, p=0.041} was the only significant variable associated with survival in patients with systemic amyloidosis. In cardiac amyloidosis, old age (≥65 years old) (HR 18.336, 95% CI 2.264-148.954, p=0.006), elevation of cTNI (HR 13.246, 95% CI 1.514-115.925, p=0.020), LV systolic dysfunction (EF <50%) (HR 5.137, 95% CI 1.065-24.770, p=0.041) and LV diastolic dysfunction (HR 64.595, 95% CI 1.834-2274.958, p=0.022) were the independent predictors of survival (Table 5).
Cardiac involvement is frequent in systemic amyloidosis and is a major determinant of the treatment options and prognosis.9) Cardiac involvement is observed in about 50% of the patients with AL amyloidosis10) and is the cause of death in approximately half of the patients with AL amyloidosis.11) And fewer than 5% of the patients with AL amyloidosis involving the heart have clinically isolated cardiac disease.12) In our study, cardiac involvement was demonstrated in 48.8% cases of systemic amyloidosis, mostly in patients with AL amyloidosis, and it was found in only 2 patients with familial amyloidosis.
The presence of monoclonal gammopathy can be demonstrated by a bone marrow biopsy showing predominance of kappa or lambda producing plasma cells or by the presence of a monoclonal light chain in the serum or urine. Between PEP and IEP/IFE/FLC assay, IEP/IFE or FLC assay is favored because of the high sensitivity compared to PEP. In the present study, we confirmed the very high sensitivity of the serum FLC assay in the diagnosis of AL amyloidosis, with a sensitivity of 96.4% in contrast to the low sensitivity of serum or urine PEP (35.8%). This finding is supported by a previous study.13) Morris et al.13) reported that in their study of 31 AL amyloidosis patients, serum and urine PEP was positive in 30%, serum IEP in 67%, urine IEP in 83%, serum and urine IEP in 90%, absolute clonal light chain ≥100 mg/L in 87%, and abnormal kappa to lambda ratio with increased clonal light chain in 97%. When a normal FLC assay result is found in a patient suspected of having cardiac amyloidosis, investigations for senile or familial amyloidosis should be included in the diagnostic work up.
Although the gold standard for the diagnosis of cardiac amyloidosis is endomyocardial biopsy, ECG is considered a key player to investigate diagnostic suspicions of cardiac amyloidosis, with low voltage QRS providing a particularly valuable noninvasive clue. Dubrey et al.12) reported that more than 70% of the patients with AL cardiac amyloidosis exhibited low voltage amplitudes, and in another series, Murtagh et al.14) found that only 46% of the patients with primary systemic amyloidosis and biopsy-proven cardiac involvement exhibited low voltage amplitudes.
The most common echocardiographic feature is thickening of the LV wall.15-18) The combination of an increased LV mass in the absence of high ECG voltages may be more specific for infiltrative diseases, of which amyloid is the most common.17)19) High sensitivity (72% to 79%) and specificity (91% to 100%) have been reported for this combination.18)19) In the present study, low voltage QRS was present in 85.5% and increased LV wall thickness was present in 96.8% of the patients.
Cardiovascular magnetic resonance imaging now has an established role in the diagnosis of cardiac involvement. Although, CMR was available in only a small number of the patients in the present study, LGE in a global or subendocardial distribution was present in 13 of 15 (86.7%) patients, similar to the study of Perugini et al.6) He reported that gadolinium enhancement by CMR was detected in 16 of 21 (76%) patients with histologically proven systemic amyloidosis and echocardiographic diagnosis of cardiac involvement.6) Subsequent studies have substantiated the diagnostic value of CMR with LGE in identifying cardiac involvement, and they suggested that the presence of LGE may confer prognostic information.5)20)
The prognosis of the amyloidosis varies, but it is generally poor if the disease is untreated. Among various prognostic factors, the extent of cardiac involvement is the most important determinant of the clinical outcome.9)21) In the present study, survival was worse in patients with cardiac involvement than those without cardiac involvement, and the 1-year, 2-year and 3-year survival of the patients with cardiac involvement was 53.2%, 46.8% and 41.9%, respectively. And 82.3% of the patients were treated with chemotherapy, 19.4% with autologous stem cell transplantation and 4.8% with cardiac transplantation. The survival rates of our study were markedly better than in the study of Kyle and Greipp,22) who reported a 1-year survival of approximately 30%, and similar to the previous study of Kristen et al.21) who reported 1- and 3-year survival of 68% and 63%, respectively. Deaths were more frequent in patients not eligible for high-dose chemotherapy and autologous stem cell transplantation. High-dose melphalan chemotherapy and autologous stem cell transplantation are generally accepted as a therapeutic approach to improve survival23) and quality of life24) in AL amyloidosis.
Our study did not show a survival benefit in the treatment group in both systemic and cardiac amyloidosis. However, to evaluate the response to chemotherapy and the prognosis, we performed a more detailed analysis of the impact of chemotherapy on survival according to the response to chemotherapy using the hematologic response criteria of Palladini et al.25) We could obtain data on the response to chemotherapy in 59.6% of patients with AL amyloidosis (complete response 19.2%, very good partial response 2.0%, partial response 12.1%, no response 26.3%) and in 57.7% of patients with AL amyloidosis with cardiac involvement (complete response 17.6%, very good partial response 3.9%, partial response 13.7%, no response 23.5%). Due to the limited data on the response to treatment in our study, we reclassified the response to chemotherapy into 2 groups; a good response group (complete response or very good partial response) and a partial or no response group. At the 3-year follow-up of patients with AL amyloidosis, the good response group showed significantly lower mortality than the partial or no response group (23.8% vs. 65.0%, p=0.003) (Fig. 7). Also in patients with AL amyloidosis with cardiac involvement, the good response group showed lower mortality than the partial or no response group (27.3% vs. 78.9%, p=0.012) (Fig. 8), a result which is concordant with previous studies.23)
In the present study, old age, elevation of cTNI, LV systolic dysfunction and diastolic dysfunction were associated with survival. Previously proposed associations with poor prognosis in cardiac amyloidosis include reduced EF, diastolic dysfunction, increased LV wall thickness on echocardiography, low voltage QRS in ECG, and the type of amyloidosis (a worse prognosis was found in the AL type compared with the TTR type).21)26) In addition, suppression of amyloidogenic serum light chains by chemotherapy, lower baseline values and greater reductions in pro-BNP, lower baseline value in cardiac troponin, and age have been associated with an improved outcome.27-29) Our data were in line with these previous findings.
There are several limitations of our study. First, given the rarity of systemic amyloidosis and cardiac amyloidosis, patient numbers were relatively small, although we reviewed 13-years' medical records from our institution. Therefore, some detailed comparisons were outside the scope of this study, and further work is needed. Second, the large loss to follow-up of our study may be a source of bias. For example, 47.3% of the patients with systemic amyloidosis and 40.3% of the patients with cardiac amyloidosis were lost to follow-up. To compensate for the high dropout rate in our study, we obtained the database of the dead collected by the Ministry of Security and Public Administration. Third, although 77.5% of the patients with systemic amyloidosis and 82.3% of the patients with cardiac amyloidosis were treated with chemotherapy, we obtained the data on the response to treatment in only 61.0% of the patients with systemic amyloidosis and 58.8% of the patients with cardiac amyloidosis. The high drop-out rate and limited data on the response to treatment might be a source of bias in the interpretation of the impact of treatment on survival in systemic and cardiac amyloidosis. Fourth, cardiac histology was only present in a subset of patients as the decision to perform a cardiac biopsy was made at the charging physicians' discretion, which may have introduced bias. Finally, although CMR shows promise for diagnosing cardiac amyloidosis if the echocardiographic features are suspicious, CMR results were only available in 24.2% of the patients with cardiac amyloidosis in our study. Therefore, some issues related to CMR could not be fully discussed.
Figures and Tables
Fig. 1
Electrocardiography in a patient with cardiac amyloidosis. Note the low voltage QRS in the limb leads.
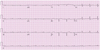
Fig. 2
Echocardiography in a patient with cardiac amyloidosis. Top left and right, parasternal long-axis and apical four chamber view demonstrates increased left ventricular wall thickening with a small amount of pericardial effusion. Bottom left and right, transmitral doppler flow shows a pseudonormalization pattern with an increased E/E' ratio.
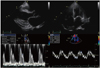
Fig. 3
Cardiovascular magnetic resonance imaging in a patient with cardiac amyloidosis. Top row shows diastolic frames from cines (vertical long axis, horizontal long axis, and short axis, respectively) showing a thickened left ventricular wall and presence of pericardial effusions (black arrow). Bottom row shows LGE images in the same planes. The vertical and horizontal long axis image demonstrates global hyperenhancement of both ventricles, both atria and the interatrial septum (white arrows). LGE: late gadolinium enhancement.
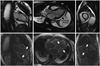
Fig. 4
Kaplan-Meier survival curves of patients with systemic amyloidosis with cardiac involvement vs. without cardiac involvement.

Fig. 5
Kaplan-Meier survival curves of patients with systemic amyloidosis with treatment vs. without treatment.

Fig. 6
Kaplan-Meier survival curves of patients with cardiac amyloidosis with treatment vs. without treatment.

Fig. 7
Kaplan-Meier survival curves of patients with AL amyloidosis with a good response vs. partial or no response to chemotherapy.

Fig. 8
Kaplan-Meier survival curves of patients with AL amyloidosis with cardiac involvement with a good response vs. partial or no response to chemotherapy.

References
1. Merlini G, Westermark P. The systemic amyloidoses: clearer understanding of the molecular mechanisms offers hope for more effective therapies. J Intern Med. 2004; 255:159–178.
2. Westermark P, Benson MD, Buxbaum JN, et al. Amyloid fibril protein nomenclature -- 2002. Amyloid. 2002; 9:197–200.
3. Duston MA, Skinner M, Shirahama T, Cohen AS. Diagnosis of amyloidosis by abdominal fat aspiration. Analysis of four years' experience. Am J Med. 1987; 82:412–414.
4. Klein AL, Hatle LK, Burstow DJ, et al. Doppler characterization of left ventricular diastolic function in cardiac amyloidosis. J Am Coll Cardiol. 1989; 13:1017–1026.
5. Vogelsberg H, Mahrholdt H, Deluigi CC, et al. Cardiovascular magnetic resonance in clinically suspected cardiac amyloidosis: noninvasive imaging compared to endomyocardial biopsy. J Am Coll Cardiol. 2008; 51:1022–1030.
6. Perugini E, Rapezzi C, Piva T, et al. Non-invasive evaluation of the myocardial substrate of cardiac amyloidosis by gadolinium cardiac magnetic resonance. Heart. 2006; 92:343–349.
7. Arbustini E, Verga L, Concardi M, Palladini G, Obici L, Merlini G. Electron and immuno-electron microscopy of abdominal fat identifies and characterizes amyloid fibrils in suspected cardiac amyloidosis. Amyloid. 2002; 9:108–114.
8. Gertz MA, Comenzo R, Falk RH, et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18-22 April 2004. Am J Hematol. 2005; 79:319–328.
9. Kyle RA, Greipp PR, O'Fallon WM. Primary systemic amyloidosis: multivariate analysis for prognostic factors in 168 cases. Blood. 1986; 68:220–224.
10. Dispenzieri A, Lacy MQ, Kyle RA, et al. Eligibility for hematopoietic stem-cell transplantation for primary systemic amyloidosis is a favorable prognostic factor for survival. J Clin Oncol. 2001; 19:3350–3356.
11. Falk RH, Skinner M. The systemic amyloidoses: an overview. Adv Intern Med. 2000; 45:107–137.
12. Dubrey SW, Cha K, Anderson J, et al. The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJM. 1998; 91:141–157.
13. Morris KL, Tate JR, Gill D, et al. Diagnostic and prognostic utility of the serum free light chain assay in patients with AL amyloidosis. Intern Med J. 2007; 37:456–463.
14. Murtagh B, Hammill SC, Gertz MA, Kyle RA, Tajik AJ, Grogan M. Electrocardiographic findings in primary systemic amyloidosis and biopsy-proven cardiac involvement. Am J Cardiol. 2005; 95:535–537.
15. Cueto-Garcia L, Tajik AJ, Kyle RA, et al. Serial echocardiographic observations in patients with primary systemic amyloidosis: an introduction to the concept of early (asymptomatic) amyloid infiltration of the heart. Mayo Clin Proc. 1984; 59:589–597.
16. Klein AL, Hatle LK, Taliercio CP, et al. Serial Doppler echocardiographic follow-up of left ventricular diastolic function in cardiac amyloidosis. J Am Coll Cardiol. 1990; 16:1135–1141.
17. Hamer JP, Janssen S, van Rijswijk MH, Lie KI. Amyloid cardiomyopathy in systemic non-hereditary amyloidosis. Clinical, echocardiographic and electrocardiographic findings in 30 patients with AA and 24 patients with AL amyloidosis. Eur Heart J. 1992; 13:623–627.
18. Rahman JE, Helou EF, Gelzer-Bell R, et al. Noninvasive diagnosis of biopsy-proven cardiac amyloidosis. J Am Coll Cardiol. 2004; 43:410–415.
19. Carroll JD, Gaasch WH, McAdam KP. Amyloid cardiomyopathy: characterization by a distinctive voltage/mass relation. Am J Cardiol. 1982; 49:9–13.
20. Syed IS, Glockner JF, Feng D, et al. Role of cardiac magnetic resonance imaging in the detection of cardiac amyloidosis. JACC Cardiovasc Imaging. 2010; 3:155–164.
21. Kristen AV, Perz JB, Schonland SO, et al. Non-invasive predictors of survival in cardiac amyloidosis. Eur J Heart Fail. 2007; 9:617–624.
22. Kyle RA, Greipp PR. Amyloidosis (AL). Clinical and laboratory features in 229 cases. Mayo Clin Proc. 1983; 58:665–683.
23. Skinner M, Sanchorawala V, Seldin DC, et al. High-dose melphalan and autologous stem-cell transplantation in patients with AL amyloidosis: an 8-year study. Ann Intern Med. 2004; 140:85–93.
24. Seldin DC, Anderson JJ, Sanchorawala V, et al. Improvement in quality of life of patients with AL amyloidosis treated with high-dose melphalan and autologous stem cell transplantation. Blood. 2004; 104:1888–1893.
25. Palladini G, Dispenzieri A, Gertz MA, et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol. 2012; 30:4541–4549.
26. Klein AL, Hatle LK, Taliercio CP, et al. Prognostic significance of Doppler measures of diastolic function in cardiac amyloidosis. A Doppler echocardiography study. Circulation. 1991; 83:808–816.
27. Lachmann HJ, Gallimore R, Gillmore JD, et al. Outcome in systemic AL amyloidosis in relation to changes in concentration of circulating free immunoglobulin light chains following chemotherapy. Br J Haematol. 2003; 122:78–84.
28. Palladini G, Lavatelli F, Russo P, et al. Circulating amyloidogenic free light chains and serum N-terminal natriuretic peptide type B decrease simultaneously in association with improvement of survival in AL. Blood. 2006; 107:3854–3858.
29. Dispenzieri A, Kyle RA, Gertz MA, et al. Survival in patients with primary systemic amyloidosis and raised serum cardiac troponins. Lancet. 2003; 361:1787–1789.
30. Nishimura RA, Tajik AJ. Evaluation of diastolic filling of left ventricle in health and disease: Doppler echocardiography is the clinician's Rosetta Stone. J Am Coll Cardiol. 1997; 30:8–18.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download