Abstract
Background and Objectives
Life-threatening hypotension during percutaneous coronary interventions (PCI) is devastating for the patient and is associated with fatal adverse outcomes. The aim of our study was to assess the usefulness of intracoronary epinephrine in severe hypotension unresponsive to other measures during PCI.
Subjects and Methods
We analyzed the Pusan National University Yangsan hospital cardiac catheterization laboratory database to identify patients who underwent PCI from December 2008 to July 2012. The outcomes were changes of blood pressure (BP) and heart rate (HR) before and after intracoronary epinephrine and in-hospital mortality.
Results
A total of 30 patients who were initially stable and received intracoronary epinephrine for severe hypotension during PCI were included. Following administration of intracoronary epinephrine (dose 181±24.8 microgram), systolic and diastolic BP (from 53.8±13.0 mm Hg up to 112.8±21.2 mm Hg, from 35±7.6 mm Hg up to 70.6±12.7 mm Hg, respectively) and HR (from 39.4±5.1 beats/min up to 96.8±29.3 beats/min) were increased. Additionally, 21 patients (70%) showed hemodynamically acceptable responses to intracoronary epinephrine without the intraaortic balloon pump and temporary pacemaker during the PCI. In-hospital mortality was 17% (n=5).
Conclusion
Although our study was small, intracoronary epinephrine was found to be well tolerated and resulted in prompt and successful recovery from severe hypotension in most patients when other measures were ineffective. Intracoronary epinephrine could be a safe and useful measure in patients developing severe hypotension during PCI.
Life-threatening hypotension or shock could occur unexpectedly during percutaneous coronary intervention (PCI). Hypotension may usually respond to traditional measures such as intravenous fluids and boluses or infusions of inotropic and vasopressor agents. When hypotension is more profound, intraaortic balloon pump (IABP), extracorporeal membrane oxygenation (ECMO), or even cardiopulmonary resuscitation may be required. These measures such as IABP and ECMO are sometimes effective on severe hypotension. However, they can be inconvenient due to difficulty in femoral puncture especially during transradial approach PCI. These measures may also be unnecessary because of transient hypotension. In such cases of severe transient hypotension, some reports have reported that intracoronary epinephrine is useful.1)2) The aim of our study was to assess the usefulness of intracoronary epinephrine in severe hypotension unresponsive to other measures during PCI.
From December 2008 to July 2012, a total of 1940 patients who underwent PCI in the Pusan National University Yangsan hospital cardiac catheterization laboratory were analyzed. Of these, 30 patients who were initially stable and receiving intracoronary epinephrine for severe hypotension during PCI were included in this study.
Our study was a retrospective database review of our experience with intracoronary epinephrine in the management of severe hypotension during PCI. Data of all patients who underwent PCI were entered into a computerized cardiac catheterization laboratory database on a daily basis. Hypotension was defined as a systolic blood pressure (BP) of less than 90 mm Hg or a 30% decrease from the baseline value. To be considered severe, hypotension had to persist despite administration of at least one pharmacologic intervention. Angiography and PCI were performed using standard techniques. All patients received 325 mg of aspirin, 600 mg of clopidogrel, intravenous heparin, and beta blockers as tolerated. Treatments for hypotension during PCI, which were intravenous fluid, atropine, boluses or infusions of inotropic and vasopressor agents, and IABP, were chosen at the discretion of the operator. The regimen of intracoronary epinephrine was two ampoules of 1 : 1000 epinephrine (1 µg/mL) mixed into 100 mL of normal saline (20 µg/mL). The dosages of intracoronary epinephrine (range, 50-200 µg) were adjusted based on the presence and severity of systemic hypotension, with larger and repeated doses for profound hypotension given accordingly (Fig. 1).
Clinical variables collected for all patients included hemodynamics, cardiac rhythm, and the timing and dosages of intracoronary epinephrine as well as other pharmacologic therapies for hypotension. Catheterization data included procedure indication, procedural details, and angiographic analysis including Thrombosis in Myocardial Infarction (TIMI) flow throughout the procedure. Outcome data included procedural success defined as ≤50% stenosis, final flow grade TIMI 3, and recovery of hemodynamics. Data regarding transvenous pacer and IABP usage as well as in-hospital mortality were collected. The main outcomes were changes of BP and heart rate (HR) before and after intracoronary epinephrine and in-hospital mortality.
The basic and clinical characteristics of all 30 patients (mean age, 63.6±11.3 years) showed higher rates of male (n=21, 70%), ST-segment elevation myocardial infarction (n=17, 56.7%) of clinical diagnosis and stable systolic BP (113.3±8.9 mm Hg), diastolic BP (67.4±13.4 mm Hg), and HR (73.6±7.9 beats/min). The main causes of severe hypotension during the PCI was no-reflow phenomenon (n=25, 83%). Other clinical and demographic characteristics are outlined in Table 1.
There were higher rates of the right coronary artery (n=17, 56%) in culprit coronary artery lesions and type C lesions (n=22, 73.4%). The majority of initial TIMI flow grade was TIMI 1 (n=10, 33%) or 2 (n=11, 37%), while the majority of post TIMI flow grade was TIMI 3 (n=21, 70%). Radial vascular access was highly performed in 73% (n=22) of these patients (Table 2).
Effects of intracoronary epinephrine on severe hypotension during PCI were summarized in Table 3. Administration of intracoronary epinephrine (mean dose, 181±24.8 µg) resulted in significant overall improvements in systolic and diastolic BP (from 53.8±13.0 up to 112.8±21.2 mm Hg, from 35±7.6 up to 70.6±12.7 mm Hg, respectively). Not surprisingly, intracoronary epinephrine resulted in significantly increased HR (from 39.4±5.1 up to 96.8±29.3 beats/min), but fortunately resulted in no cases of acute dysrhythmia.
Additionally, IABP was required in 7 (24%) patients, both IABP and temporary pacing in 1 (3%) patient, and ECMO in 1 (3%) patient due to persistent severe hypotension even after intracoronary epinephrine injection. Therefore, 21 patients (70%) showed hemodynamically acceptable responses to intracoronary epinephrine without the IABP and temporary pacemaker during the PCI. In-hospital mortality was 17% (n=5) (Table 1).
The aim of this study was to assess the usefulness of intracoronary epinephrine in severe hypotension unresponsive to other measures during PCI. Our data showed that administration of intracoronary epinephrine resulted in significant improvements in systolic and diastolic BP (from 53.8±13.0 mm Hg up to 112.8±21.2 mm Hg, from 35±7.6 mm Hg up to 70.6±12.7 mm Hg, respectively) in almost all patients and restored normal BP in the majority of cases. Moreover, intracoronary epinephrine was safely administered without significant adverse effects.
Epinephrine has been the primary drug for in the purpose of resuscitation from cardiac arrest.3-5) Epinephrine is a mixed potent adrenergic agonist, acting on alpha- and beta-adrenergic receptors. 2)5) At low doses, beta-adrenergic effects (vasodilatation) on the vascular system predominate, whereas at high doses, alpha-adrenergic effects (vasoconstriction) increase as the dose increases.3)6) Epinephrine administered at low concentrations (e.g., 0.1 µg/kg) paradoxically can produce vasodilation because low dose epinephrine have greater sensitivity to vasodilator beta-2 receptors than vasoconstrictor alpha receptors,3)7) and beta-adrenergic stimulation leads to coronary vasodilatation.8-10) However, high dose epinephrine increases myocardial oxygen consumption, decrease myocardial ATP with prodysrhythmic effects such as ventricular fibrillation and myocardial lactate level.4)6)7) Because there were some limitations regarding dose-dependent effects of epinephrine, we had to use epinephrine carefully.
Moreover, different effects of epinephrine have been noted depending on its administration routes. The traditional routes of administration such as venous route have some potential disadvantages. Lower concentrations of the inotropic agent reach the heart and systemic circulation because the venous route could have the disequilibrium dilution effect and pulmonary elimination.11) Additionally, venous administration of epinephrine may trigger pulmonary vasoconstriction, resulting in increased pulmonary arterial pressure and pulmonary vascular resistance.12) Aral et al.12) evaluated the hemodynamic advantages of left atrial epinephrine administration during open heart operation. They showed that the average pulmonary arterial pressure and pulmonary vascular resistance were higher in the central venous group, whereas higher cardiac indices and average BP were noted in the left atrial group. They concluded that epinephrine infusion through the left atrial route is associated with greater hemodynamic advantages than infusion through the central venous route.
Life-threatening hypotension during PCI, resulting from acute bleeding, coronary perforation with cardiac tamponade, arrhythmia, contrast- or medication-induced anaphylaxis, no reflow phenomenon, coronary spasm, and new thrombus formation, could occur unexpectedly and be associated with fatal adverse outcomes. Hypotension usually responds to traditional measures such as intravenous fluids, atropine and boluses or infusions of inotropic and vasopressor agents. When the hypotension is more profound, an IABP, ECMO may be required. These measures such as IABP and ECMO are sometimes effective on severe hypotension. However, they are usually unnecessary because hypotension during PCI is often transient. Moreover, these measures are not easy to perform especially during transradial PCI because femoral puncture is usually difficult due to low BP. As intracoronary epinephrine can restore systolic and diastolic BP and acquire the time for preparation of other interventions such as temporary pacemaker, IABP and ECMO, intracoronary epinephrine can reduce the need of unnecessary interventions and be a "pharmacologic bridge" to definitive intervention.
Some studies have reported that intracoronary epinephrine is useful on severe transient hypotension.1)2) In our study, the main causes of severe hypotension during the PCI was no-reflow phenomenon (n=25, 83%). Intracoronary administrations of nitroglycerine, nitroprusside, verapamil, adenosine, and Glycoprotein IIb/IIIa inhibitors have been reported as traditional pharmacologic therapies. However, success rates are variable and in some cases no-reflow is refractory to multiple agents.1) Due to its inotropic and vasodilator effects, intracoronary epinephrine has been considered for the treatment of no-reflow.13) Skelding et al.1) evaluated the effects of intracoronary epinephrine (bolus range, 50-200 µg; mean dose, 139.8-189 µg) in the setting of refractory no-reflow; intracoronary epinephrine resulted in an overall improvement (69% of PCI) in coronary flow, restoration of BP in hypotensive patients and increased HR in the absence of major tachyarrhythmias. In our study, despite the no-reflow phenomenon refractory to traditional agents, intracoronary epinephrine constantly improved coronary flow and did not infrequently reverse no-reflow. An improvement in coronary flow was established with beneficial effects on BP in patients exhibiting hypotension with no-reflow. It has been suggested that intracoronary epinephrine has a potential role in ischemic patients with no-reflow and hypotension.1)2)10) However, randomized trials on the use of epinephrine are lacking. Besides the useful effect of no-reflow phenomenon, intracoronary epinephrine may be the last resort in the case of refractory hypotension due to contrast- or medication-induced anaphylaxis, coronary dissection, coronary spasm, and new thrombus formation during PCI.
There is no consensus about the safe dosage of intracoronary epinephrine in patients with refractory hypotension during the PCI. Only two clinical reports mentioned dosage of intracoronary epinephrine (range 50-400 µg) by arbitrarily selection,1)2) but no cases of acute dysrhythmia were noted.
Nonetheless, the present study has several limitations. First, our study was a small, observational and non-randomized single center study. Second, we excluded patients with missing data, which may reflect selection bias. Thus, to fully evaluate long-term clinical outcomes, large-scale long-term prospective randomized trials are needed in the future.
Intracoronary epinephrine may have useful effects such as inotropic, chronotropic and coronary vasodilator effects in patients developing severe hypotension during PCI. Although our study was small, intracoronary epinephrine was well tolerated and resulted in prompt and successful recovery from severe hypotension in most patients when other measures were ineffective. Intracoronary epinephrine could be a safe and useful measure in patients developing severe hypotension during PCI.
Figures and Tables
Fig. 1
The regimen of intracoronary epinephrine. The regimen of intracoronary epinephrine was two ampoules of 1 : 1000 epinephrine (1 µg/mL) mixed into 100 mL of normal saline (20 µg/mL).

Table 1
Baseline clinical characteristics
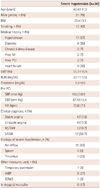
All values are means±SD. BMI: body mass index, MI: myocardial infarction, PCI: primary coronary intervention, LVEF: left ventricular ejection fraction, BUN: blood urea nitrogen, BP: blood pressure, NSTEMI: non-ST elevation myocardial elevation, STEMI: ST-elevation myocardial infarction, IABP: intraaortic balloon pump, ECMO: extracorporeal membrane oxygenation
Acknowledgments
This work was supported for two years by a Pusan National University Research Grant.
References
1. Skelding KA, Goldstein JA, Mehta L, Pica MC, O'Neill WW. Resolution of refractory no-reflow with intracoronary epinephrine. Catheter Cardiovasc Interv. 2002; 57:305–309.
2. Kahn JK, Hartzler GO. Reversal of refractory hypotension with intracoronary epinephrine during coronary angioplasty. Am Heart J. 1993; 126:463–466.
3. Brunton LL, Chabner BA, Knollmann BC. Adrenergic agonists and antagonists. In : Brunton LL, editor. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 12th ed. McGraw-Hill;2010. p. 282–287.
4. Otto CW, Yakaitis RW. The role of epinephrine in CPR: a reappraisal. Ann Emerg Med. 1984; 13(9 Pt 2):840–843.
5. Zhong JQ, Dorian P. Epinephrine and vasopressin during cardiopulmonary resuscitation. Resuscitation. 2005; 66:263–269.
6. Kitsou V, Xanthos T, Stroumpoulis K, et al. Nitroglycerin and epinephrine improve coronary perfusion pressure in a porcine model of ventricular fibrillation arrest: a pilot study. J Emerg Med. 2009; 37:369–375.
7. Grmec S, Mally S. Vasopressin improves outcome in out-of-hospital cardiopulmonary resuscitation of ventricular fibrillation and pulseless ventricular tachycardia: a observational cohort study. Crit Care. 2006; 10:R13.
8. Robertson RM, Wood AJ, Vaughn WK, Robertson D. Exacerbation of vasotonic angina pectoris by propranolol. Circulation. 1982; 65:281–285.
9. Kiss G, Corre O, Gueret G, et al. Management of cardiac arrest caused by coronary artery spasm: epinephrine/adrenaline versus nitrates. Heart Lung. 2009; 38:228–232.
10. Baim DS. Epinephrine: a new pharmacologic treatment for no-reflow? Catheter Cardiovasc Interv. 2002; 57:310–311.
11. Comroe JH. Nonrespiratory functions of the lungs and circulation. Physiology of respiration. 2nd ed. Chicago: Year Book Medical Publishers;1974. p. 285–282.
12. Aral A, Oğuz M, Ozberrak H, et al. Hemodynamic advantages of left atrial epinephrine administration in open heart operations. Ann Thorac Surg. 1997; 64:1046–1049.
13. Lucreziotti S, Sponzilli C, Castini D, Di Domenico E, Fiorentini C. Intracoronary epinephrine for contrast-medium-induced microvascular obstruction in a chronically hemodialyzed patient. Cardiology. 2005; 103:196–198.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download