Abstract
Glycoprotein IIb/IIIa antagonists are well established for their effectiveness in improving clinical outcomes in acute coronary syndrome patients undergoing percutaneous coronary intervention. Acute profound thrombocytopenia is a rare complication of abciximab. We present a case which was managed successfully for the rare complication of acute profound thrombocytopenia after using abciximab and an intra-aortic balloon pump for the treatment of a no-reflow phenomenon and consecutive cardiogenic shock during primary percutaneous coronary intervention.
In patients undergoing percutaneous coronary intervention (PCI) for the treatment of acute coronary syndrome, administration of glycoprotein (GP) IIb/IIIa receptor antagonists has been shown to improve their clinical outcomes. However, the associated hemorrhagic risk may evoke serious complications, such as acute profound thrombocytopenia.1) We present a successfully managed case of a rare complication of acute profound thrombocytopenia after the use abciximab, an intra-aortic balloon pump (IABP) for the treatment of no-reflow phenomenon, and consecutive cardiogenic shock during primary PCI in a patient with ST-segment elevated myocardial infarction (STEMI).
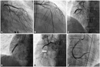
A 65 year-old man with hypertension visited the local hospital with resting chest pain for 3 hours. He was transferred to our hospital due to the abnormal electrocardiogram (ECG) findings showing an elevated ST-segment in the inferior wall territory with a reciprocal change, which was suspicious of STEMI. In the emergency room, blood pressure was 140/80 mm Hg and chest radiography showed no definite abnormal findings, which was graded as Killip class 1. It was decided to have the patient undergo primary PCI for the treatment of STEMI. Conventional medical treatments that include a bolus injection of 5000 IU of heparin, a loading dose of aspirin (300 mg), and clopidogrel (300 mg) was applied before primary PCI. Coronary angiography (CAG) showed a totally occluded lesion of the proximal right coronary artery (RCA) with collateral flow grade 1 from the left anterior descending artery (Fig. 1A, B, and C). After predilatation with a 2.0×20 mm conventional balloon, a bare metal stent 5.0×24 mm Liberte® (Boston Scientific, Natick, MA, USA) was implanted in the proximal RCA lesion (Fig. 1D). After implantation of a stent, no-reflow phenomenon developed (Fig. 1E) and blood pressure decreased with findings of a complete atrioventricular-block and re-elevation of the ST-segment on ECG monitoring. The patient was treated with intracoronary injection of nitrate and adenosine, and intravenous bolus consecutive injection of abciximab with maintenance (intravenous bolus of 0.25 mg/kg, 10 to 60 minutes during the procedure, followed by 0.125 µg/kg/min infusion for 12 hours). Although an improvement was observed in the Thrombolysis in Myocardial Infarction flow grade from grade 0 to grade 3 (Fig. 1F) and the patient returned to sinus rhythm, the hypotension was sustained. Therefore, an IABP was inserted into the left femoral artery. The patient was moved to the coronary care unit for intensive monitoring with maintenance of abciximab and IABP.
Although the baseline platelet count was 167000/µL before PCI, a routine check determined a complete blood count showing a decreased platelet count of 6000/µL at 22 hours after bolus administration of abciximab, followed by a platelet count of 3000/µL 4 hours later. It was considered that the patient had acute, profound thrombocytopenia as a complication of abciximab. Heparin induced thrombocytopenia (HIT) was excluded because HIT typically develops after 6 to 10 days of heparin use with no previous exposure to heparin. In this case, the patient had no history of exposure to heparin. Therefore, the patient was started on clopidogrel 75 mg/day and aspirin 100 mg/day were started to prevent stent thrombosis. The patient was administered 4 units of platelet concentrates as a precaution. The patient was withdrawn from heparin treatment in consideration of additional hemorrhagic risk, but not HIT. The next followed count of platelet increased to 24000/µL without clinical evidence of stent thrombosis, such as chest pain and a change of ST-segment on ECG. Vital signs were stabilized the next day and IABP was removed successfully using the closing device, Perclose® (Abbott, Redwood City, CA, USA) due to the hemorrhagic risk of the access site and a decreased platelet count of 26000/µL. After additional transfusions of 4 units of platelet concentrates, the platelet count increased to 49000/µL without complications. Subsequently, the platelet count rose to 164000/µL on discharge (Fig. 2). A 1-year follow up of CAG showed good flow of RCA without chest pain or thrombocytopenia.
The benefit of platelet inhibition by GP IIb/IIIa inhibitors, as adjunctive treatment in patients undergoing PCI, is well established. Abciximab, a potent antiplatelet agent that blocks the final pathways to platelet aggregation,2) improves the outcomes of high risk procedures and decreases the incidence of major adverse cardiac events. However, the associated hemorrhagic risk may be a major safety concern. According to previous studies, the incidence of thrombocytopenia (platelet count of <100000/mm3) is 0.5% to 5.6%.3-5) In particular, acute profound thrombocytopenia, defined as a platelet count of <20000/mm3, is a rare and serious complication, since it increases the risk for significant bleeding. However, an increased risk of stent thrombosis, due to the need for antithrombotic drugs discontinuation and platelet transfusion should be considered to determine the proper therapeutic approach.
Although little is known about the pathophysiological mechanisms that lead to thrombocytopenia induced by GP IIb/IIIa antagonists, preexisting platelet surface antibodies and GP IIb/IIIa antagonists may induce the immune response of platelets by conformational changes in the GP IIb/IIIa receptors on the platelet surface and the expression of new epitopes recognized by the antibodies.6)
The platelet count is necessary to detect thrombocytopenia, and should be done before starting treatment with abciximab, 1-4 hours later and, if it decreases, once a day until resolution (for platelet count <20000/mm3 it should be checked twice a day until the count rises to 50000/mm3). Several drugs, such as heparin and clopidogrel, could induce thrombocytopenia, thus should also be considered as possible causative factors. It is important to exclude HIT, because both heparin and abciximab are frequently infused.7) There is no test that can distinguish this syndrome from HIT. However, abciximab induced thrombocytopenia occurs rapidly after the administration of abciximab, while HIT develops over a period of days in patients without previous exposure to heparin. HIT is not acute and it may be associated with bleeding and thrombosis.8) In this case, thrombocytopenia was identified the next day after administration of abciximab and heparin without a previous exposure history of heparin. Therefore, HIT could be excluded. Earlier and frequent monitoring of the platelet count is warranted.
Acute profound thrombocytopenia as a complication of abciximab is a more problematic situation in this case. It can create hemorrhagic complications. However, the following should be considered: microvascular thromboembolic condition of no-reflow phenomenon with poor clinical outcomes, such as a lack of recovery of the left ventricular function of affected myocardial segments, increased in-hospital mortality, a poor long-term prognosis,9-11) and the need for additional usage of heparin for the maintenance of IABP. In addition, the management of abciximab-induced profound thrombocytopenia is controversial, since there is no previous evidence of this in the medical literature. Therefore, clopidogrel 75 mg/day and aspirin 100 mg/day were started and only heparin was withdrawn. In addition, it was difficult to expect a value of transfusion of platelet concentrates in the condition of no-reflow phenomenon. Transfusion of platelet concentrates was decided based on the operator's experience in the present case.
In summary, we report a case of a 65-year-old man with a rare complication of acute profound thrombocytopenia after administration of abciximab for the treatment of no-reflow phenomenon during primary PCI. Although HIT should be differentiated and the removal of IABP was an issue due to the risk of increased bleeding, our patient was managed successfully with transfusion of platelet concentrates.
Figures and Tables
Fig. 1
Findings of coronary angiography and primary percutaneous coronary intervention. A and B: no specific stenosis in the left coronary artery. C: a totally occluded lesion of the proximal RCA with collateral flow grade 1 from LAD. D: a bare metal stent was implanted in the proximal RCA lesion. E: after implantation of a stent, the coronary angiogram shows no-reflow phenomenon. The arrowindicates the implanted stent. F: improvement of TIMI flow grade from grade 0 to grade 3 after intracoronary injection of nitrate and adenosine, and intravenous injection of abciximab. LAD: left anterior descending artery, RCA: right coronary artery, TIMI: Thrombolysis in Myocardial Infarction.
References
1. Makoni SN. Acute profound thrombocytopenia following angioplasty: the dilemma in the management and a review of the literature. Heart. 2001; 86:E18.
2. Coller BS. Blockade of platelet GPIIb/IIIa receptors as an antithrombotic strategy. Circulation. 1995; 92:2373–2380.
3. Use of a monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor in high-risk coronary angioplasty. The EPIC Investigation. N Engl J Med. 1994; 330:956–961.
4. Lincoff AM, Mark DB, Tcheng JE, et al. Economic assessment of platelet glycoprotein IIb/IIIa receptor blockade with abciximab and low-dose heparin during percutaneous coronary revascularization: results from the EPILOG randomized trial. Evaluation in PTCA to Improve Long-term Outcome with abciximab GP IIb/IIIa blockade. Circulation. 2000; 102:2923–2929.
5. EPISTENT Investigators. Randomised placebo-controlled and balloon-angioplasty-controlled trial to assess safety of coronary stenting with use of platelet glycoprotein-IIb/IIIa blockade. Lancet. 1998; 352:87–92.
6. Merlini PA, Rossi M, Menozzi A, et al. Thrombocytopenia caused by abciximab or tirofiban and its association with clinical outcome in patients undergoing coronary stenting. Circulation. 2004; 109:2203–2206.
7. Warkentin TE. Heparin-induced thrombocytopenia: pathogenesis and management. Br J Haematol. 2003; 121:535–555.
8. Claeys LG, Berg W. Major bleeding and severe thrombocytopenia after combined heparin and abciximab-c7E3 Fab therapy. Eur J Vasc Endovasc Surg. 2003; 25:85–87.
9. Klein LW, Kern MJ, Berger P, et al. Society of cardiac angiography and interventions: suggested management of the no-reflow phenomenon in the cardiac catheterization laboratory. Catheter Cardiovasc Interv. 2003; 60:194–201.
10. Butler MJ, Chan W, Taylor AJ, Dart AM, Duffy SJ. Management of the no-reflow phenomenon. Pharmacol Ther. 2011; 132:72–85.
11. Abbo KM, Dooris M, Glazier S, et al. Features and outcome of no-reflow after percutaneous coronary intervention. Am J Cardiol. 1995; 75:778–782.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download