Abstract
Background and Objectives
Abdominal Aortic Aneurysm (AAA) and carotid disease have medical and social significance, considering their morbidity, disability, and economic consequences. The study objectives were to determine the prevalence of asymptomatic internal carotid artery (ICA) lesions ≥70% in patients with AAA, the correlation of AAA diameter with the degree of ICA stenosis and symptoms, and the importance of preventive ultrasound checkups.
Subjects and Methods
A prospective non-randomized controlled study including 740 patients, aged from 18-85 years, who were suitable for the inclusion and exclusion criteria and reported at the vascular laboratory of the Institute for Vascular and Endovascular Surgery, Clinical Center of Serbia from 1st of December 2011 to the 1st of November 2012.
Results
The prevalence of asymptomatic ICA stenosis ≥70% in patients with AAA is 10.8%. Male representatives have more symptomatic ICA stenosis ≥70%. Patients with small aneurysms more often have asymptomatic ICA stenosis ≥70%. The occurrence of symptoms of carotid disease was more prevalent among patients with ICA stenosis ≥70% compared to the group with stenosis <70%. There was no correlation found between the grade of ICA stenosis with the size of AAA.
Conclusion
The prevalence of asymptomatic ICA stenosis ≥70% in patients with AAA is found to be 10.8%. Male patients with ICA stenosis ≥70% more often had symptoms of carotid disease. In the smaller aneurysms, ICA stenosis ≥70% occurs frequently, but without the symptoms of carotid disease, and there was no correlation between the size of AAA and the grade of ICA stenosis. Clinical implications of ICA imaging in patients with previously diagnosed AAA is necessary.
Heart and blood vessel diseases are the leading causes of death and disability worldwide. An additional problem with these patients is the fact that they have an insidious course of disease progress, because they are asymptomatic for a long time. The first manifestation is often a life-threatening condition {myocardial infarction, stroke, ruptured Abdominal Aortic Aneurysm (AAA)}. Screening procedures, primarily duplex ultrasonography as non-invasive diagnostic procedures, could help in the early detection of potentially dangerous pathological conditions.
In the sixteenth century, anatomist Vesalius1) presented the first description of AAA, and in 1817 Sir Cooper Astely performed the first attempted surgical treatment of AAA. The first modern surgery of AAA was performed by Dubost et al.,2) a French surgeon, in 1951. The further development of AAA surgery enabled the work of the creators of modern cardiovascular surgery, Americans De Bakey and Cooley.3) Parodi et al.,4) a radiologist from Argentina, presented the endovascular treatment of AAA in 1991.
Predisposing factors for their occurrence are: smoking (AAA is eight times more frequent among smokers than among nonsmokers),5) years of age (the incidence of AAA is 5% higher among men older than 65 years of age),6) gender (there is a four times higher incidence among males than females),7) hyperlipidemia, hypertension, family tendency (AAA occurs 4-6 times more frequently in male relatives with the risk of rupture of 20-30%). Occurrence of AAA also varies depending on geographic area (English 4.7%, while among Asian people it is around 0.45%).8) AAA is also less frequent in people of African and Spanish origin.
The natural course of AAA leads to complications. They are compression (inferior vena cava, duodenum, roots of spinal nerves), thrombosis (rarely completely due to the high flow, pressure, and diameter). However, parts of the clot easily and often produce distal embolisation and rupture, as the most significant complication.
Up to the 1990s, arteriography (translumbal, transfemoral) was used as the "gold standard" for the diagnosis of AAA, but today the diagnosis of AAA is made using modern technical facilities: ultrasound diagnostics, computed tomography, and nuclear magnetic resonance.
This study shows that 25% of patients with ruptured AAA die before reaching hospital, and 51% die in hospital without having a procedure performed. Of those remaining who undergo surgery, the mortality rate is 46% and the overall 30-day survival rate is only 11%.9) According to worldwide data, the risk of rupture is dependent on the size of the aneurysm. Aneurysms of a diameter of 40-49 mm have a risk of rupture of 0.5-5% annually, a 50-59 mm diameter a risk of 3-15%, and a diameter of 60-69 mm a 10-20% risk of rupture.6)
Atherosclerosis is a chronic, systemic, degenerative-inflammatoryproliferative disease. It develops primarily in large and medium arteries, with predilection at their bifurcation. It is also a ubiquitous disease (it is spread across all races, in all meridians) and begins developing at an early age, with increasing tendency with age and other risk factors (smoking, diabetes mellitus, a positive history of cardiovascular disease and electrocardiography abnormalities, hypertrophy of the left ventricular, atrial fibrillation, arterial hypertension, dyslipoproteinemia, hyperhomocysteinemia, and psycho-physical passivity, etc.). In 1951, Fisher10) first described the symptoms and pathology of atherosclerotic carotid artery disease. The prevalence of significant asymptomatic carotid stenosis in the general population varies from 0% to 3.1%, which is useful to know in any discussion of the cost-effectiveness of screening for carotid artery stenosis.11) Stenosis over 50% are encountered in the population in 6-11% of men over 60 years and 5-7% of women.12) Patients with asymptomatic carotid artery lesions have a risk of stroke of 1.5% per year and 7.5% per five years.13) The risk is higher immediately after the Transient Ischemic Attack (TIA), and continues to be about 5% during the first few months after the TIA. Of all patients with TIA, 20-25% are estimated to develop a stroke within two years. Steno-occlusive disease of the carotid arteries can cause a TIA, amaurosis fugax, Reversible Ischemic Neurologic Deficit, and various forms of stroke.
The first reconstructive intervention on carotid arteries was performed in Buenos Aires in 1951 (Carré, Mollins and Murphy). Strully et al.,14) in 1953, after an unsuccessful attempt of carotid endarterectomy, performed ligation and resection of the internal carotid artery (ICA). The first eversion carotid endarterectomy was announced by De Bakey et al. in 1953. The patient was with complete occlusion.15)
During the surgical treatment of AAA, undiagnosed hemodynamic significant carotid artery stenosis can cause stroke. Therefore, before any treatment of AAA we must treat hemodynamic significant asymptomatic carotid arteries.16) It is also important to understand the prevalence of this phenomenon.
The objectives of this study were:
- Determination of the prevalence of asymptomatic hemodynamic significant stenosis of carotid artery in patients with AAA
- The correlation between the diameter of AAA on the degree of ICA stenosis and symptoms of carotid disease in these patients
- Importance of preventive ultrasound checkups
The prospective non-randomized controlled study included 740 patients. The study was conducted in the non-invasive ultrasound laboratory at the Department of Vascular and Endovascular Surgery, Clinical Center of Serbia in the period from 1st of December 2011 to the 1st of November 2012. All patients had undergone a physical examination by the vascular surgeon who suspected the presence of AAA before an ultrasound was performed.
Criteria for inclusion in the study were patient age of 18 to 85 years (both sexes), in which AAA was verified by an ultrasound (patients were prepared for the exam-which implies that at least 12 hours before the examination they had a meal, a probe of 3.5 MHz, and measurements in the longitudinal and transverse section from outer to outer wall). All patients agreed to participate in the study by signing a consent form, and were suitable for planning surgical or endovascular treatment.
Exclusion criteria were: all patients with AAA who had been diagnosed earlier and had symptoms of carotid disease, patients who had contraindications for surgical treatment or for whom endovascular treatment was impossible because of morphological reasons, as well as previously urgently treated ruptured AAA patients. Patients underwent an ultrasound exam of carotid arteries. The examination was performed with a linear transducer frequency of 7-10 MHz. The patient was lying on their back, head turned to the opposite of the examining side. The degree of stenosis was determined on the basis of peak systolic velocity (PSV), end-diastolic velocity, and PSV ratio measurements and cross-area stenosis-mentioned consensus documents of San Francisco since 2002 were examined.
Applying these criteria, in order to meet the objectives of the study, the examination included 740 patients with complete data, on the basis of which we made a further descriptive and statistical analysis.
III 1. Monitoring of variables
- Medical history variables (age, sex)
III 2. Statistical analysis of data
All collected data were analyzed using modern methods of descriptive and analytic statistics and the computer aided software package Statistical Package for the Social Sciences (SPSS) 12.0 (SPSS Inc., Chicago, IL, USA). Statistical processing and analysis was performed using SPSS ver. 12.0, and a graphical and tabular presentation was conducted using the Microsoft Office suite of products (Excel, Word, and later PowerPoint).
The following descriptive statistical methods were used:
Tabulation, calculating measures of central tendency: mean, median, mode, calculating a measure of variability: standard deviation
The following analytic statistical methods were used:
- Fisher's exact test, Mann-Whitney U test, chi-square test, Student t-test
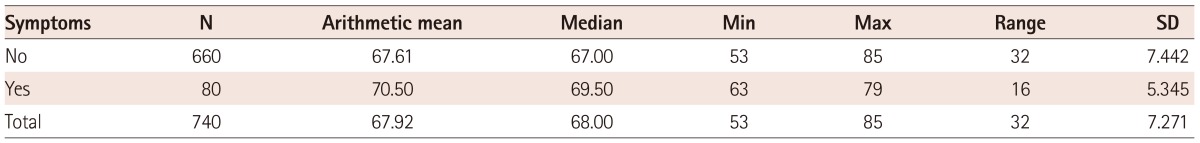
The mean age of patients who were examined was 68 years. The oldest patient was 85 years old, and the youngest 53 years. There were 650 (88%) male patients and 90 (12%) were females. Of the 740 patients who had been included in the study, 660 were asymptomatic. Their average age was 68 years (youngest 53 and the oldest 85), while the number of patients with symptoms was 80. Their average age was 70 years (the youngest 63 and the oldest was 79 years old) (Table 1 and 2b).
Based on the t-test (t=1064, df=72, p>0.06) the age difference was not statistically significant in terms of symptoms.
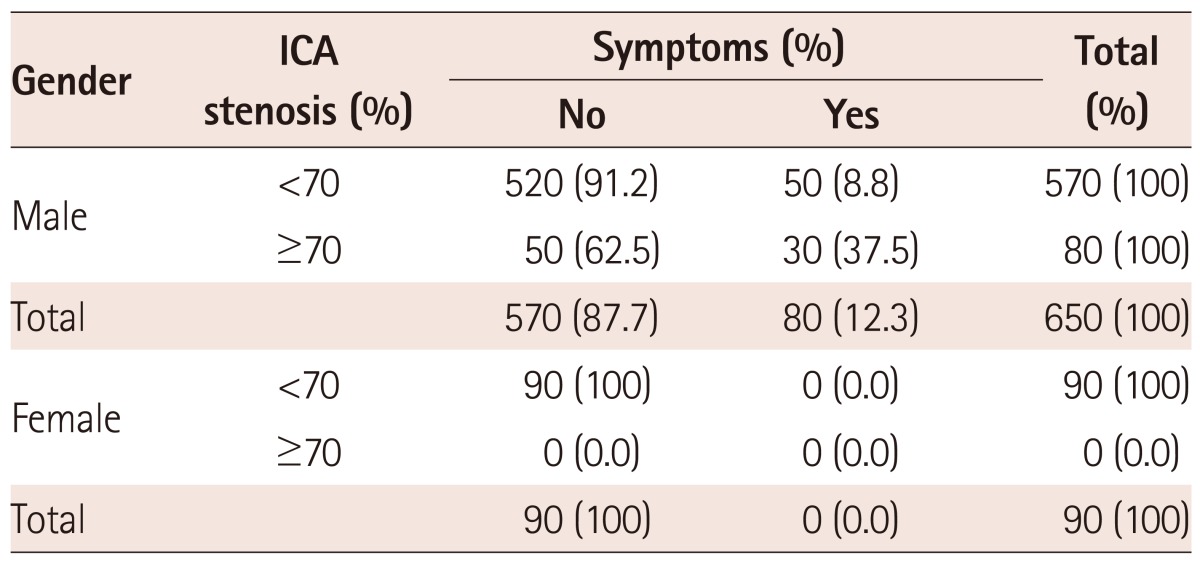
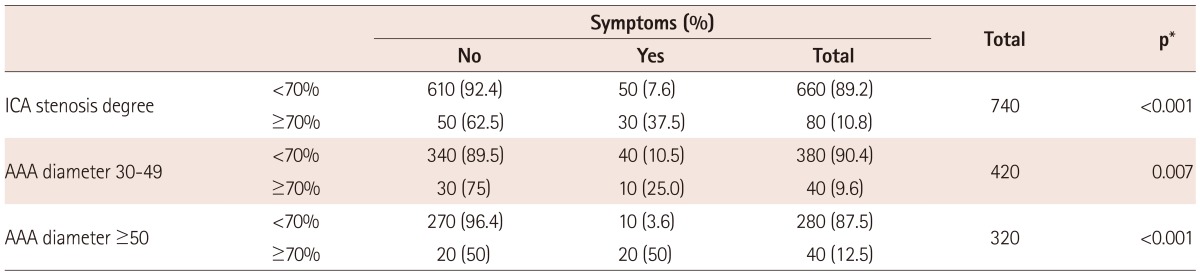
From among the 570 male patients who had ICA stenosis less than 70%, 50 (8.8%) of them had symptoms. 50 male patients had asymptomatic stenosis equal to or greater than 70% and 30 had symptoms (37.5%). Among the female patients, the observed stenosis <70% was found in 90 patients, and there was no case of ICA stenosis greater than 70%. All female patients were asymptomatic. The statistical analysis by the Fisher exact test, which was 0052, showed a statistically significant difference in favor of males. This means that male patients with significant ICA stenosis, more often than females, had symptoms of carotid disease (Table 2a).
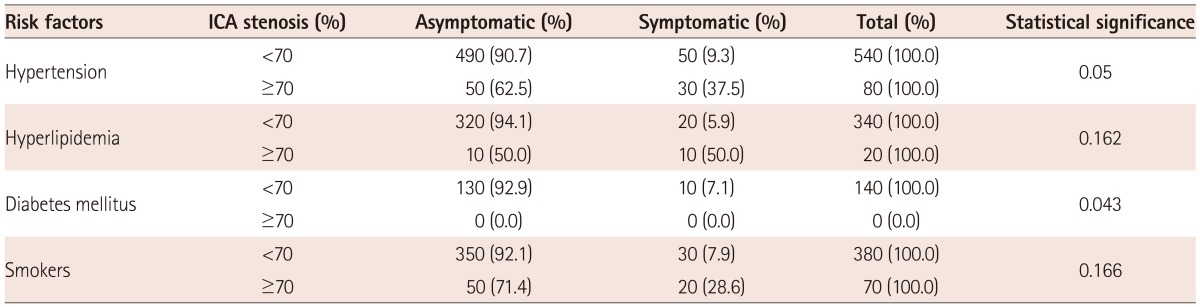
From the risk factors in observed groups, the only statistically significant factor was hypertension. Other risk factors (hyperlipidemia, diabetes mellitus and smoking) did not demonstrate statistical significance.
Table 4 shows that in the group of patients with smaller AAA there were 30 patients (75%) with asymptomatic significant hemodynamic ICA lesions and 10 (25%) were symptomatic. Therefore, the group of patients with small aneurysm had ICA stenosis 70% more frequently, but without symptoms of carotid disease. In the group with large aneurysms, there were observed 20 patients (50%) in each subgroup (asymptomatic/symptomatic hemodynamic significant ICA stenosis).
Both small and large AAA have the same number (40 : 40, 9.6% : 12.5%) of high grade ICA stenosis (ICA stenosis ≥70%), suggesting that there is no correlation between grade of ICA stenosis and size of AAA (40/420, 9.6% vs. 40/320, 12.5%).
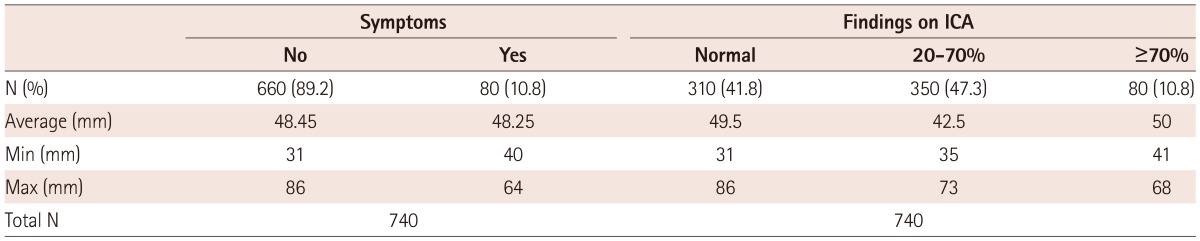
Three hundred ten patients (41.8%) had normal findings on ICA with the average size of AAA in those patients of 49.5 mm, a minimum of 31 mm and a maximum of 86 mm. 350 (47.3%) patients with 20-70% stenosis of ICA had AAA with an average diameter of 42.5 mm (35-73 mm). ICA stenosis greater than 70% numbered 80 patients (10.8%) and the average size of their AAA was 50 mm (minimum 41 mm and maximum 68 mm). There were no differences in the average size of AAA and the appearance of symptoms of ICA stenosis (asymptomatic 48.45 : symptomatic 48.25).
According to the Mann-Whitney U test, Z=-0009, p=0.993, and p>0.05, so there were no statistically significant differences observed (Table 5).
Asymptomatic disease is a significant problem in clinical practice, especially in a population that is not medically enlightened and is not accustomed to having regular checkups. Asymptomatic ICA stenosis and AAA are serious illnesses and the time when they become symptomatic can be life threatening for patients.
The incidence of asymptomatic AAA ranges from 3-8%.17) The incidence of asymptomatic ICA stenosis depends on patient age, ranging from 0.5-10%.
The treatment of asymptomatic patients is complex, in terms of the choices of therapeutic procedures (medication, surgical, endovascular) and also deciding on the right moment for the procedures.
Today, it is widely accepted that surgical intervention or endovascular procedures on AAA are required when the diameter is greater than 5 cm or if the annual growth rate is larger than 0.6 mm, if it is eccentric or secular and if stress increases the pressure inside the aneurysm.
Predisposing factors for the occurrence of TIA and stroke are certainly atherosclerotic lesions in the carotid arteries. The frequency depends on the degree of ICA stenosis, its morphology, and other factors as hypertension, smoking, sex, and age of the patient.
Several important studies {NASCET, ECST, Asymptomatic Carotid Artery Surgery (ACAS), Asymptomatic Carotid Surgery Trial (ACST)}18)19) have attempted to address concerns related to the incidence of symptomatic and asymptomatic stenosis, the occurrence of TIA and stroke, and what might be the most appropriate therapy for this problem.
Fayad20) analyzed three major studies of asymptomatic carotid stenosis. The ACAS and ACST and Veterans Affairs Cooperative Study Group trial concluded that carotid endarterectomy reduces the absolute risk of stroke by 5.4-5.9% for 5 years, and at the same time, the preoperative risk of stroke and death was 2.3-4.7%.
In conclusion, Pierre believes that people younger than 80 years without comorbidity with low surgical risk, with moderate or severe ICA stenosis have an increased risk of stroke and death by 12% over five years. In patients with asymptomatic ICA stenosis, the annual risk of stroke ranges from 1.3-3.3%. The risk increases with a higher degree of stenosis.
Norris et al.21) in his work shows that the TIA and stroke occur in 10.5% of patients with stenosis ≥75%. Carotid endarterectomy in these patients reduces the absolute risk of stroke and death by 5-6%, and the relative risk is reduced by 50%.
Chambers and Norris22) analyzed work on this topic and came to the conclusion that the preoperative stroke or mortality risk was noted at 3%. Carotid endarterectomy reduces the risk of stroke by 30% in a three-year period. However, the absolute risk reduction is small, at about 1% per year.
In his work, Bertine23) found that the prevalence of asymptomatic ICA stenosis ≥50% was the greatest in patients with peripheral vascular disease, at 15%, while the prevalence in AAA was 12%.
Cabellon found that of 66 patients with asymptomatic stenosis of ICA 10.6% have AAA, which was confirmed by ultrasound. Our results were similar to the results of these two authors. The prevalence of asymptomatic hemodynamic significant stenosis of ICA in patients with AAA was found to be 10.8%.
In the general population, the prevalence of ICA stenosis ranges from 13-30%. The incidence increases with the patient's age. Clinically significant asymptomatic carotid stenosis defined as stenosis ≥50% have lower prevalence rates and ranges from 1.5-9%.22-26) In older people, it occurs in about 28% of cases, as published in the Swedish study.
In the North American Cardiovascular Health Study27) and the Framingham study, the prevalence ranges from 5-7% in women and 7-9% in men. The risk of stroke in symptomatic ICA stenosis increases three times, for asymptomatic stenosis it is slightly lower and ranges from 1-1.5% on an annual basis.
The data published in the NASCET indicates that more than 45% of all ischemic strokes were caused by asymptomatic carotid stenosis. The presence of such a large percentage of non-symptomatic strokes can be explained by the presence of adequate intracerebral collateral circulation.
The presence of coronary artery disease, hypertension, AAA, and periphery vascular disease in asymptomatic carotid stenosis is a significant problem and a high-risk group in clinical practice. It is shown that stroke in coronary surgery occurs in a small percentage of less than 2%, but in the group of patients with asymptomatic stenosis, it occurs in about 8%. The frequency of asymptomatic ICA stenosis in the general population increases with age. In people younger than 50 years old, the incidence of asymptomatic ICA stenosis was 0.5%, and among those older than 65 years, from 5-10%.
Although age is an important predictor for the prevalence of ICA stenosis, in our study, we have not observed a statistically significant difference between ages in the groups with and without symptoms, probably due to minor differences in the years, since the average asymptomatic group age was 68, and the symptomatic age was 70 years (Table 1). The age difference is not statistically significant in terms of symptoms.
In contrast to this, we found a statistically significant sex differences. Male patients with significant ICA stenosis, more often than females, had symptoms of carotid disease (Table 2a).
Other authors (Framingham study) found the same results in favor of the male gender.
A recent study found that 36% of patients who are known to have AAA also have significant carotid artery disease.
Kang et al.28) found that the risk of AAA is 2-3 times higher in patients with carotid artery stenosis then in the general population.
Young has described in his work a relationship between the prevalence of significant carotid disease and AAA.
Bengtsson observed that AAA diameter in patients with carotid artery disease increased rapidly. Smaller aneurysms are increased annually by 0.8 mm and aneurysms greater than 4 cm by 3.3 mm, suggesting that patients with AAA should undergo frequent ultrasound examinations in order to prevent the rupture of the AAA.
In our paper from the risk factors in observed groups, statistical significant was only hypertension. Other risk factors (hyperlipidemia, diabetes mellitus, and smoking) did not have any statistical significance on symptoms and grade of ICA stenosis (Table 3).
In our results we see that in the group of patients with an AAA diameter equal to or greater than 50 mm the same percentage (50%) of symptomatic and asymptomatic hemodynamic significant carotid artery lesions was detected. While in the group of small aneurysms (AAA 30-49 mm) we observed a higher percentage of asymptomatic hemodynamic significant ICA lesions, 75% compared to 25% (Table 4). Comparing whether patients have or have not symptoms of ICA disease, we concluded that in patients with small AAA there are more frequent patients without symptoms of ICA stenosis, even when it is greater than 70%.
Furthermore, from Table 4 we concluded that there is no correlation between AAA size and grade of ICA stenosis (40/420, 9.6% vs. 40/320, 12.5%).
From Table 5 on the basis of our results we also concluded that the size of AAA and the degree of stenosis do not correlate, as in Table 4. The group of patients without any atherosclerotic plaque in the ICA had an average diameter of 49.5 mm of AAA, compared to 50 mm, which was reported in the group with high-grade ICA stenosis (70%). Interestingly, the maximum AAA diameter of 86 mm in all three groups was found in patients without atherosclerotic plaque in ICA.
Some studies showed that the reduction of ABI for 0.2 units increases the cause of death from cardiovascular disease by 28%.
Zureik et al.29) in his paper showed that the cumulative value of death from cardiovascular disease was significantly higher in patients with ICA stenosis ≥50%.
Liapis et al.30) in his work observed these results: from patients that previously had surgery of AAA, 75% of them also had ICA stenosis greater then 50%, and 25% had stenosis ≥70%. This finding agrees with our opinion that routine ultrasound screening of ICA should be perform in patients with AAA.
In conclusions, the prevalence of asymptomatic hemodynamic significant stenosis of the ICA in patients with AAA is found in 10.8% of the population.
Male patients with ICA stenosis greater then 70%, more often than females, had symptoms of carotid disease.
In the smaller aneurysms, ICA stenosis greater than 70% occurs frequently, but without symptoms of carotid disease.
There is no correlation between the size of the AAA and the grade of ICA stenosis.
On the basis of previous findings it is necessary to perform a preventive ultrasound of a carotid artery in patients who had been previously diagnosed with AAA.
References
1. Leonardo RA. History of Surgery. New York: Froben Press;1943.
2. Dubost C, Allary M, Oeconomos N. Resection of an aneurysm of the abdominal aorta: reestablishment of the continuity by a preserved human arterial graft, with result after five months. AMA Arch Surg. 1952; 64:405–408. PMID: 14894065.
3. De Bakey ME, Cooley DA. Surgical treatment of aneurysm of abdominal aorta by resection and restoration of continuity with homograft. Surg Gynecol Obstet. 1953; 97:257–266. PMID: 13090050.
4. Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg. 1991; 5:491–499. PMID: 1837729.
5. Wilmink TB, Quick CR, Day NE. The association between cigarette smoking and abdominal aortic aneurysms. J Vasc Surg. 1999; 30:1099–1105. PMID: 10587395.
6. Multicentre Aneurysm Screening Study Group. Multicentre aneurysm screening study (MASS): cost effectiveness analysis of screening for abdominal aortic aneurysms based on four year results from randomised controlled trial. BMJ. 2002; 325:1135. PMID: 12433761.
7. Lederle FA, Johnson GR, Wilson SE. Aneurysm Detection and Management Veterans Affairs Cooperative Study. Abdominal aortic aneurysm in women. J Vasc Surg. 2001; 34:122–126. PMID: 11436084.
8. Salem MK, Rayt HS, Hussey G, et al. Should Asian men be included in abdominal aortic aneurysm screening programmes? Eur J Vasc Endovasc Surg. 2009; 38:748–749. PMID: 19666232.
9. Brown PM, Pattenden R, Vernooy C, Zelt DT, Gutelius JR. Selective management of abdominal aortic aneurysms in a prospective measurement program. J Vasc Surg. 1996; 23:213–220. discussion 221-2. PMID: 8637098.
10. Fisher M. Occlusion of the internal carotid artery. AMA Arch Neurol Psychiatry. 1951; 65:346–377.
11. Clinical advisory: carotid endarterectomy for patients with asymptomatic internal carotid artery stenosis. Stroke. 1994; 25:2523–2524. PMID: 7974602.
12. Lee TT, Solomon NA, Heidenreich PA, Oehlert J, Garber AM. Cost-effectiveness of screening for carotid stenosis in asymptomatic persons. Ann Intern Med. 1997; 126:337–346. PMID: 9054277.
13. Wiebers DO, Whisnant JP, Sandok BA, O'Fallon WM. Prospective comparison of a cohort with asymptomatic carotid bruit and a populationbased cohort without carotid bruit. Stroke. 1990; 21:984–988. PMID: 2368113.
14. Strully KJ, Hurwitt ES, Blankenberg HW. Thrombo-endarterectomy for thrombosis of the internal carotid artery in the neck. J Neurosurg. 1953; 10:474–482. PMID: 13097208.
15. De Bakey ME, Crawford ES, Cooley DA, Morris GC Jr. Surgical considerations of occlusive disease of innominate, carotid, subclavian, and vertebral arteries. Ann Surg. 1959; 149:690–710. PMID: 13637687.
16. Zwibel WJ, Pellerito JS. Introduction to vascular ultrasonography. 5th ed. Philadelphia: Elsevier Saunders;2004. p. 272.
17. Sila CA, Higashida RT, Clagett GP. Clinical decisions. Management of carotid stenosis. N Engl J Med. 2008; 358:1617–1621. PMID: 18403770.
18. Risk of stroke in the distribution of an asymptomatic carotid artery. The European Carotid Surgery Trialists Collaborative Group. Lancet. 1995; 345:209–212. PMID: 7823712.
19. Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA. 1995; 273:1421–1428. PMID: 7723155.
20. Fayad P. Endarterectomy and stenting for asymptomatic carotid stenosis: a race at breakneck speed. Stroke. 2007; 38(2 Suppl):707–714. PMID: 17261722.
21. Norris JW, Zhu CZ, Bornstein NM, Chambers BR. Vascular risks of asymptomatic carotid stenosis. Stroke. 1991; 22:1485–1490. PMID: 1962321.
22. Chambers BR, Norris JW. The case against surgery for asymptomatic carotid stenosis. Stroke. 1984; 15:964–967. PMID: 6506125.
23. Goessens BM, Visseren FL, Kappelle LJ, Algra A, van der. Asymptomatic carotid artery stenosis and the risk of new vascular events in patients with manifest arterial disease: the SMART study. Stroke. 2007; 38:1470–1475. PMID: 17363718.
24. Willeit J, Kiechl S. Prevalence and risk factors of asymptomatic extracranial carotid artery atherosclerosis. A population-based study. Arterioscler Thromb. 1993; 13:661–668. PMID: 8485116.
25. O'Leary DH, Polak JF, Kronmal RA, et al. Distribution and correlates of sonographically detected carotid artery disease in the Cardiovascular Health Study. The CHS Collaborative Research Group. Stroke. 1992; 23:1752–1760. PMID: 1448826.
26. Pujia A, Rubba P, Spencer MP. Prevalence of extracranial carotid artery disease detectable by echo-Doppler in an elderly population. Stroke. 1992; 23:818–822. PMID: 1595098.
27. Alcorn HG, Wolfson SK Jr, Sutton-Tyrrell K, Kuller LH, O'Leary D. Risk factors for abdominal aortic aneurysms in older adults enrolled in The Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1996; 16:963–970. PMID: 8696960.
28. Kang SS, Littooy FN, Gupta SR, et al. Higher prevalence of abdominal aortic aneurysms in patients with carotid stenosis but without diabetes. Surgery. 1999; 126:687–691. discussion 691-2. PMID: 10520916.
29. Zureik M, Temmar M, Adamopoulos C, et al. Carotid plaques, but not common carotid intima-media thickness, are independently associated with aortic stiffness. J Hypertens. 2002; 20:85–93. PMID: 11791030.
30. Liapis CD, Kakisis JD, Dimitroulis DA, Daskalopoulos M, Nikolaou A, Kostakis AG. Carotid ultrasound findings as a predictor of long-term survival after abdominal aortic aneurysm repair: a 14-year prospective study. J Vasc Surg. 2003; 38:1220–1225. PMID: 14681618.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download