Abstract
A 65 year-old female with a history of xerostomia and xerophthalmia was presented with dyspnea on exertion (New York Heart Association class III). Echocardiography and cardiac catheterization demonstrated severe pulmonary hypertension (PH). Laboratory examinations showed positive anti-nuclear and anti-Ro/SS-A antibodies. Schirmer's test was positive and salivary gland scintigraphy revealed severely decreased tracer uptakes in both parotid and submandibular glands. By excluding other possible causes of PH during further examinations, she was diagnosed with severe PH associated with primary Sjögren's syndrome. Her dyspnea symptom was much improved with endothelin receptor antagonist and azathioprine.
Pulmonary manifestations in patients with primary Sjögren's syndrome include a variety of interstitial lung disease, airway disease, pleurisy, lymphoma, pseudolymphoma, amyloidosis, pulmonary vasculitis, granulomatous disease, and diaphragmatic myopathy.1) Although pulmonary hypertension (PH) can be accompanied with primary Sjögren's syndrome, this kind of pulmonary manifestation is more frequently associated with other connective tissue diseases (CTD).2-7) Several cases of PH in patients with primary Sjögren's syndrome have been reported in some English publications.8-11) However, there has been no reported case in Korea until now.
We report the first Korean case of PH developed in a patient with primary Sjögren's syndrome.
A 65-year-old female was presented with dyspnea on exertion (New York Heart Association, NYHA class III) for over a period of 1 year. For 5 years, she had complained of dry mouth, intermittent dry eye, and gritty feeling under the eyelids. However, she did not have Raynaud's phenomenon or parotid swelling. She denied taking anorexiants or other drugs. Further, she did not have any sort of family history.
Blood pressure, pulse rate, and respiration rate were 114/78 mm Hg, 92 per minute, and 24 per minute, respectively. Cardiovascular examination revealed a grade 2/6 systolic murmur in the left low parasternal area, a more accentuated P2 than A2, and both jugular veins that are both slightly distended.
Laboratory exam disclosed polyclonal hypergammaglobulinemia identified by slightly increased total protein (8.2 g/dL, normal 6.5-8.1 g/dL) and gamma globulin (22.4%, normal 11.1-18.8%). Autoantibody tests showed positive results for anti-nuclear antibodies (1 : 640, speckled, anticytoplasmic antibody: positive), rheumatoid factors (57.10 IU/mL, normal <14 IU/mL), and anti-Ro (SSA) antibodies (3+), while anti-neutrophil cytoplasmic antibodies (ANCA), antibodies to La (SSB), double stranded DNA, Sm, ribonucleoprotein (RNP), topoisomerase-I (Scl-70), histidyl-tRNA synthetase (Jo-1) were negative. Antibodies to cardiolipin and lupus anticoagulant were also negative. Serum complements protein levels were all slightly decreased (C3 87.0 mg/dL, normal 90-180 mg/dL; C4 7.2 mg/dL, normal 10-40 mg/dL; CH50 21.6 U/mL, normal 34-71 U/mL). Anti-human immunodeficiency virus antibodies, hepatitis B virus antigen and antibodies, and anti-hepatitis C antibodies were all negative. Erythrocyte sedimentation rate was increased (42 mm/hour, normal 0-20 mm/hour). Hemoglobin, white blood cell and platelet counts, and urine test were within normal limits. N-terminal pro B-type natriuretic peptide level was 1469.1 pg/mL (normal 0-125 pg/mL). Creatinine kinase-MB and troponin-I were also within normal limits. Arterial blood gas analysis in room air showed pH 7.44, pO2 78 mm Hg, and pCO2 34 mm Hg.
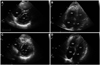
Pulmonary function test revealed normal ventilatory pattern with normal CO diffusion capacity. Chest X-ray demonstrated grossly normal lung fields with a mildly enlarged right ventricle. Electrocar-diogram showed tall, peak P waves in lead II, III, and aVF, suggesting right atrial enlargement. Transthoracic echocardiography showed a mildly enlarged and thick right ventricle, hypokinetic right ventricular free wall, grade II tricuspid regurgitation, and peak tricuspid regurgitation jet velocity of 4.64 m/s with an estimated right ventricular systolic pressure (RVSP) of 91.1 mm Hg (Fig. 1A and B). Transthoracic and transesophageal echocardiography excluded any other congenital, valvular, and myocardial diseases. Right heart catheterization also revealed severe PH. Pulmonary artery pressure was 74/27 mm Hg (mean 46 mm Hg) with a pulmonary capillary wedge pressure of 7 mm Hg (Fig. 2). Cardiac index was calculated as 2.27 L/min/m2 and pulmonary vascular resistance was 11 Wood units. Elevated pulmonary artery pressure was not significantly decreased with adenosine infusion. Chest computed tomography and lung perfusion scan did not show abnormal findings compatible with pulmonary embolism. Schirmer's test was positive. Salivary gland scintigraphy disclosed severely decreased tracer uptakes in both parotid and submandibular glands.
Considering her clinical, laboratory, cardiologic, imaging findings and the Revised International Classification Criteria for Sjögren's Syndrome by the American-European Consensus Group,12) she was clearly diagnosed with severe PH associated with primary Sjögren's syndrome. She was treated with 62.5 mg endothelin receptor antagonist (Bosentan®) twice a day for the initial month and 125 mg twice a day for the following months, 200 mg hydroxychloroquine twice a day, 50 mg azathioprine twice a day, and topical corticosteroid and sodium hyaluronidate eye drops. A follow-up transthoracic echocardiography after 6 months of treatment showed somewhat improved right ventricle contractility and decreased estimated RVSP (65.5 mm Hg) compared to the initial exam (Fig. 1C and D). Equally, her dyspnea symptom was much improved and uneventfully, she is being followed up at an outpatient clinic.
Sjögren's syndrome is a chronic inflammatory autoimmune disease characterized by lymphocytic infiltration at the exocrine glands and at other extraglandular sites resulting in the dryness of the mouth and eyes. The disease predominantly affects women in the fourth and fifth decades of life, with a female to male ratio of 9 : 1. It can be either primary or secondary to another CTD, most commonly rheumatoid arthritis. Clinical manifestations of the disease vary from exocrinopathy, such as salivary or ocular involvement (keratoconjunctivitis sicca), less commonly respiratory tree or gastrointestinal tract involvement to systemic extraglandular manifestations, such as arthritis, Raynaud's phenomenon, lung, kidney involvement, vasculitis, or lymphoma.13) Among them, pulmonary manifestations in primary Sjögren's syndrome include a variety of diffuse interstitial lung disease, airway disease, lymphoma, pseudolymphoma, amyloidosis, pleural involvement, vasculitis, and PH.1) PH is associated with several CTDs, most commonly with scleroderma,2) its limited cutaneous variant, the Calcinosis, Raynaud phenomenon, Esophageal dysmotility, Sclerodactyly, Telengiectasis (CREST) syndrome,3)4) mixed connective tissue disease (MCTD),5) and systemic lupus erythematosus (SLE).6)7) However, the prevalence of PH in primary Sjögren's syndrome is rare throughout the world and there has been no case report in Korea until now.
The pathogenesis of PH in primary Sjögren's syndrome is uncertain; however, it is thought result from vasculitis with prolonged vasospasm followed by structural vessel remodeling, eventually leading to the irreversible thrombotic obstruction of pulmonary arterioles.1) Launay et al.11) revealed that compared with primary Sjögren's syndrome patients without pulmonary artery hypertension (PAH), primary Sjögren's syndrome patients with PAH experienced Raynaud phenomenon, cutaneous vasculitis, and interstitial lung disease more frequently. They were more likely to have anti-nuclear, anti-Ro/SSA, and anti-RNP autoantibodies, as well as positive rheumatoid factors and hypergammaglobulinemia. These data imply that systemic vasculopathy, B-cell activation, and autoimmunity are involved in the pathogenesis of PAH associated with primary Sjögren's syndrome, supporting that immunosuppressants play a vital role in the treatment of PAH in primary Sjögren's syndrome.11)
In the treatment of PAH associated with primary Sjögren's syndrome, there has been no best treatment strategy due to the small number of accumulated cases. However, taking into consideration the facts that PAH is the main mechanism of CTD-PH, and that histopathological similarities and shared pathogenic mechanisms exist between idiopathic PAH and PAH associated with connective tissue disease (CTD-PAH), treatment for idiopathic PAH may be applicable to CTD-PAH.11)14) Oxygen should be supplemented if hypoxemia exists, and diuretic therapy may be used to reduce the right ventricular preload. Long-term anticoagulant associated with improved survival in idiopathic PAH15) can be applied, although its efficacy has not been documented in patients with PAH due to other causes, including CTDs.14) Effective pulmonary vasodilators should be administered to reduce the afterload on the right heart. Calcium-channel blockers should be used for responders to short-acting vasodilator challenge test during right cardiac catheterization. However, it is well-documented that this therapy is effective in less than 10% of patients with idiopathic PAH. Furthermore, in patients with CTD-PAH, the percentage of non-responders to calcium-channel blockers is higher than that in patients with idiopathic PAH.16) Several drugs targeting the mechanism of PAH have been developed. Prostacyclin analogues, such as Epoprostenol, Treprostinil, Iloprost, which are potent vasodilators, can be used. Endothelin receptor antagonists, such as Bosentan and Ambrisentan, can also be applied by the fact that endothelin, which has powerful vasoconstrictive effects is increased in PAH. Bosentan was approved in the EU and the USA for the treatment of idiopathic PAH and CTD-PAH in patients who have NYHA class III dyspnea. The third is nitric oxide pathway targeted therapy. Phosphodiesterase-5 inhibitors, such as Sildenafil and Vardenafil, block the degradation of cyclic guanosine monophosphate, which is the second messenger in nitric oxide-induced pulmonary vasodilation.11)14) In addition to these standard PAH therapies, corticosteroids and/or other immunosuppressants would also be used in CTD-PAH, considering that immune or inflammatory mechanisms play an important role in the genesis or progression of PAH, particularly in CTD-PAH.17) Pulse steroid therapy in PAH associated with MCTD has been suggested to be effective, resulting in the improvement of hemodynamic parameters and dyspnea functional class.18) Furthermore, it was proved that intermittent pulse therapy with cyclophosphamide was effective in mild to moderate PAH associated with SLE.19)
Although there had been no available randomized controlled data with regard to PAH associated with other CTDs, Launay et al.11) suggested the treatment algorithm of PAH associated with primary Sjögren's syndrome. They proposed that initial immunosuppressive therapy (cyclophosphamide or azathioprine) should be taken in patients with NYHA class I/II dyspnea and immunosuppressive therapy; additionally, standard PAH therapy (endothelin receptor antagonists, phosphodiseterase-5 inhibitors or prostanoids) should be given to patients with NYHA class III/IV dyspnea.
The long term prognosis in the majority of patients with CTD-PAH is known to be worse than that in patients with idiopathic PAH. In addition, the survival rates in patients with PAH associated with primary Sjögren's syndrome were also poor, according to the accumulated data. Therefore, accurate diagnosis and early effective treatment with immunosuppressants and/or standard PAH therapy are very important in patients with PAH associated with primary Sjögren's syndrome.
Figures and Tables
Fig. 1
Initial transthoracic echocardiography demonstrates a dilated right ventricle (RV) and a slightly compressed left ventricle (LV) with a flattened ventricular septum (A and B). After 6 months of treatment, a follow-up exam shows that the RV cavity was decreased in size and the ventricular septum is deviated to the RV normally (C and D). RA: right atrium, LA: left atrium.
References
1. Kokosi M, Riemer EC, Highland KB. Pulmonary involvement in Sjögren syndrome. Clin Chest Med. 2010; 31:489–500.
2. Ungerer RG, Tashkin DP, Furst D, et al. Prevalence and clinical correlates of pulmonary arterial hypertension in progressive systemic sclerosis. Am J Med. 1983; 75:65–74.
3. Salerni R, Rodnan GP, Leon DF, Shaver JA. Pulmonary hypertension in the CREST syndrome variant of progressive systemic sclerosis (scleroderma). Ann Intern Med. 1977; 86:394–399.
4. Sanchez O, Humbert M, Sitbon O, Nunes H, Garcia G, Simonneau G. [Pulmonary hypertension associated with connective tissue diseases]. Rev Med Interne. 2002; 23:41–54.
5. Wiener-Kronish JP, Solinger AM, Warnock ML, Churg A, Ordonez N, Golden JA. Severe pulmonary involvement in mixed connective tissue disease. Am Rev Respir Dis. 1981; 124:499–503.
6. Kim KH, Jeong MH, Kim W, et al. A case of systemic lupus erythematosus with severe pulmonary hypertension and pericarditis. Korean Circ J. 2000; 30:605–610.
7. Quismorio FP Jr, Sharma O, Koss M, et al. Immunopathologic and clinical studies in pulmonary hypertension associated with systemic lupus erythematosus. Semin Arthritis Rheum. 1984; 13:349–359.
8. Nakagawa N, Osanai S, Ide H, et al. Severe pulmonary hypertension associated with primary Sjögren's syndrome. Intern Med. 2003; 42:1248–1252.
9. Sowa JM. The association of Sjögren's syndrome and primary pulmonary hypertension. Hum Pathol. 1993; 24:1035–1036.
10. Sato T, Matsubara O, Tanaka Y, Kasuga T. Association of Sjögren's syndrome with pulmonary hypertension: report of two cases and review of the literature. Hum Pathol. 1993; 24:199–205.
11. Launay D, Hachulla E, Hatron PY, Jais X, Simonneau G, Humbert M. Pulmonary arterial hypertension: a rare complication of primary Sjögren syndrome: report of 9 new cases and review of the literature. Medicine (Baltimore). 2007; 86:299–315.
12. Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002; 61:554–558.
13. Kassan SS, Moutsopoulos HM. Clinical manifestations and early diagnosis of Sjögren syndrome. Arch Intern Med. 2004; 164:1275–1284.
14. Distler O, Pignone A. Pulmonary arterial hypertension and rheumatic diseases--from diagnosis to treatment. Rheumatology (Oxford). 2006; 45:Suppl 4. iv22–iv25.
15. Fuster V, Steele PM, Edwards WD, Gersh BJ, McGoon MD, Frye RL. Primary pulmonary hypertension: natural history and the importance of thrombosis. Circulation. 1984; 70:580–587.
16. Sanchez O, Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary hypertension secondary to connective tissue diseases. Thorax. 1999; 54:273–277.
17. Sanchez O, Sitbon O, Jaïs X, Simonneau G, Humbert M. Immunosuppressive therapy in connective tissue diseases-associated pulmonary arterial hypertension. Chest. 2006; 130:182–189.
18. Kamata Y, Nara H, Sato H, Masuyama JI, Minota S, Yoshio T. Effect of steroid pulse therapy on mixed connective tissue disease with pulmonary arterial hypertension. Ann Rheum Dis. 2005; 64:1236–1237.
19. Gonzalez-Lopez L, Cardona-Muñoz EG, Celis A, et al. Therapy with intermittent pulse cyclophosphamide for pulmonary hypertension associated with systemic lupus erythematosus. Lupus. 2004; 13:105–112.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download